Abstract
Coronaviruses are terrifically precise and adapted towards specialized respiratory epithelial cells, observed in organ culture and human volunteers both. This virus is found to possess an unpredictable anti-viral T-cell response which in turn results in T-cell activation and finally apoptosis, leading to cytokine storm and collapse of the whole immune system. The present review provides comprehensive information regarding SARS-CoV-2 infection, mutant strains, and the impact of SARS-COV-2 on vital organs, the pathophysiology of the disease, diagnostic tests available, and possible treatments. It also includes all the vaccines developed so far throughout the world to control this pandemic. Until now, 18 vaccines have been approved by the WHO and further 22 vaccines are in the third trial. This study also provides up-to-date information regarding the drugs repurposed in clinical trials and the recent status of allopathic drugs along with its result. Although vaccines are available, specific treatment is not available for the disease. Furthermore, the effect of vaccines on new variants is a new area of research at this time. Therefore, a preventive attitude is the best approach to fight against this virus.
Similar content being viewed by others
Introduction
December 2019 has been documented as a historic month for the emergence of the coronavirus disease also called viral pneumonia in Wuhan, China. This outbreak has propounded to approx. 220 countries, possessing more than 180,906,466 confirmed cases with 3,919,082 confirmed deaths and recovered cases 165,531,010 all over the world until the 25th of June 2021.
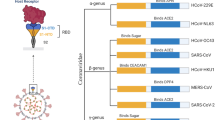
The virus was primarily cautiously named 2019 novel coronavirus (2019-nCoV). Consequently, the International Committee of Taxonomy of Viruses coined the name SARS-CoV-2, and so the disease was named COVID-19(Zhou et al. 2020; Chen et al. 2020). This virus has been classified in genus, subgenus, and family: β-coronavirus, Sarbecovirus, and Coronaviridae respectively (Fig. 1) (Zhang et al. 2020a; Pottoo et al. 2021).
SARS-CoV-2, extremely contagious and lethal, became an immense global health issue. As per the gene sequence analysis, SARS-CoV-2 is pertinent with two SARS-like coronaviruses observed in bats (approx.88% resemblance) that possess rapid spreading characteristics all through the world. There are seven strains of coronavirus detected and identified which infect humans (HCoV), four of which are HCoV-229E, HCoV-NL63, HCoV-HKU1, and HCoV-OC43 that are common, seasonal, and liable to cause mild respiratory symptoms, whereas another two are zoonotic and virulent called MERS-CoV and SARS-CoV-1 and this SARS-CoV-2 possesses genetic similarity with SARS-CoV-1, both belonging to Sarbecovirus subgenus and β-coronavirus genus (Chen et al. 2020). Further study revealed that virus possesses envelope, +ve sense, single-strand of RNA linked with 30-kb genome (Arabi et al. 2020). It possesses proofreading machinery in order to reduce the mistakes during replication, therefore the mutations.
Research on SARS-CoV-2 revealed that it corresponds to Beta-coronavirus that possesses a characteristic genome structure as shown in Fig. 1. This virus is the biggest known RNA virus that has a specialty in possessing a replication-transcription system (RTS). This also possesses 3′-5′ exo-ribonuclease (some RNA-transforming enzymes with approx. 148 proteins). The mechanism of infection in a human cell has been illustrated in Fig. 2 (Cevik et al. 2020).
Decoding of genome results into structural (four proteins, i.e., spike, membrane, envelope, and nucleocapsid) as well non-structural proteins (for replicase transcriptase proteins). It binds to ACE2 receptors through spike protein for cell attachment and entry. The disease shows flu-like symptoms such as fever, cough, loss of taste, diarrhea, pneumonia, renal, asthenia, cardiac symptoms, and skin rashes. Happy hypoxia is also observed in COVID-19-positive cases which is also responsible for the causalities. In some situations, the patient also feels acute traumatic stress disorder, depression, and some psychological disorders, and few cases commit suicide also due to it (Markham and Keam 2018). The effect of SARS Cov-2 on various vital organs is shown in Fig. 3.
Coronavirus strains
The coronavirus term implies a huge virus’s family known since a long time ago. Before this pandemic, many coronaviruses are known to infect humans and cause mild respiratory illnesses such as cough and colds. This novel COVID-19 virus is not new to us but it is very rare that a virus jumps from animals/plants to humans and causes disease. This happens with the COVID-19 virus; therefore, it infects humans rapidly, due to its extreme contagious nature (Jena 2021; Jha et al. 2021;Jin et al. 2020;Shahab et al. 2021, Huang et al. 2020). Variants known to date are compiled in Table 1.
Mutations
It is well known that viruses are the link between living and non-living organisms as they do not contain any cells. Therefore, there are more chances of mutations in viruses during human infection as well as disease progressions as the simplest structure. Most of the mutations are harmful to the organisms but few may be beneficial too. As a result of mutations, these viruses may appear in a little bit different form and can cause the disease in an extreme contagious manner as shown in the case of the COVID-19 virus.
D614G was found to be the first variant that has been observed near late January or early February 2019 and replaced the original strain somewhere in June 2020 which became dominant and spread throughout the world. The enhanced infectivity and transmission have been observed without a change in the severity of the disease.
Cluster 5, another mutant, has been observed somewhere between August and September 2020 through Danish personnel infected by framed mink which later jumped to humans. As the name suggests, Cluster 5 is an aggregate of strains, observed in 12 persons until now, not found to spread extensively. SARS-CoV-2 VOC 202012/01 (mutant of interest) has been reported by UK personnel to the WHO (December 14, 2020). This mutant possesses 23 nucleotide changes found to be not related phylogenetically to the SARS-CoV-2 virus, the main cause of coronavirus disease at that time. Due to the above substitutions, this strain was found to possess enhanced transmissibility without any change in disease severity (Ruan et al. 2020). The variants that are known and the different mutants along with the site of mutations are summarized in Table 2.
Triple COVID-19 mutation
Triple mutation in COVID-19 has also been observed in few parts of Delhi, West Bengal, and Maharashtra. Mutations at sites 452 and 484 have been reported individually; for example, in California, variants B.1.427 and B.1.429 have the L452R mutation. E484K has been seen in the variants reported from the UK, South Africa, and Brazil. Mutations at site P681 have also been seen in some lineages before as well as the mutation E484Q. The combination of all the three mutations reported here, L452R, E484Q, and P681R, suggests the virus emerging similar traits autonomously, continually adapting to its human hosts (Perrella et al. 2020; Del Rio et al. 2020).
Pathogenesis of disease
When a virus enters in body through the nose or mouth, it stays near the paranasal cavity and tries to make colonies, which results in headache and loss of smell and taste at the very first level. After 24–36 h, it invades the respiratory and gastrointestinal system and causes abdominal pain and loss of motion. The body defense system activates, and macrophages accumulate at the site and try to engulf the virus and send the messages in a form of IL-1, IL-6, and TNF to other body cells as well as neutrophils to be active against infection.
By the examining IL-6 level, we can make an idea regarding the body’s preparation against the virus. As the disease progresses, further neutrophils accumulate and try to kill the virus, but body cells will also be affected. At 5–6 days of the disease, chances of cytokine storm occur due to over responsiveness of the body. The virus attacks on type 2 pneumocytes, which secrets some surfactants and helps in lung expansion during respiration. Another target for the virus is the liver and kidney. Macrophage activates the liver to produce a c-reactive protein which gives signals to other parts of the body to be overreactive against infection (Jain 2020).
These signals can also act on the liver to produce and release fibrinogen which when reacts with platelets forming unnecessary clots in the body which floats in the blood and can block any artery/vein that may result in pulmonary embolism, heart attack, and stroke. These blood clots can be examined by testing the level of d-driver. When an infection invades the heart cells, it releases HSCRP which causes damage to the cardiac muscle cells. Cytokines can activate the thermo regulators in the hypothalamus and result in fever. The liver function and cell damage can be checked by examining SGOT, SGPT, blood level. Cell damage can be checked through LDH level and a patient’s life can be saved by taking therapy on time (Favalli et al. 2020).
Once SARS-CoV-2 enters the alveolus, it begins to infect type II alveolar cells and replicate. The infected type II alveolar cells release pro-inflammatory cytokines, which signal the immune system to respond. Patients may experience mild symptoms, such as cough, fever, and body aches. Macrophages release IL-1, IL-6, and TNF-I. IL-6, causing vasodilation and allowing more immune cells to travel to the alveolus. It also increases capillary permeability, causing plasma to leak into the interstitial space and the alveolus. Neutrophils release reactive oxygen species and proteinases, which destroy infected cells. These dead cells combine with the plasma to form a protein-rich fluid that accumulates within the alveolus, causing shortness of breath and pneumonia. Accumulation of fluid and dilution of surfactant lining of the alveolus cause alveolar collapse, which decreases gas exchange and can lead to hypoxemia and acute respiratory distress syndrome. If the immune system goes into overdrive, inflammation can spread throughout the circulatory system, leading to systemic inflammatory response syndrome, also known as a cytokine storm. This systemic inflammation can cause septic shock, where blood pressure drops dangerously low and organs can no longer be perfused, leading to multi-organ failure and death.
COVID-19-related comorbidities
As explained in the preceding sections, the SARS-Cov-2 virus has undergone numerous changes, resulting in more dangerous variants. In type 2 asthmatic individuals, COVID-19 infection becomes more severe. They also outline the treatment options and medications that can be utilized to address mild, moderate, and severe COVID-19 symptoms in asthmatic patients, preventing aggravation. The progressive results were highly contradictory, as severe cases of COVID-19 showed an increase in the levels of numerous cytokines that might exacerbate bronchial tract inflammation, exacerbating asthma attacks. Contrary to popular belief, certain data show that COVID-19 severity is reduced in type 2 asthmatic patients with elevated T-cells, since most COVID-19 positive individuals have a significant drop in T-cells. This helps to restore the balance of immunological responses, slowing the advancement of the disease (Ghosh et al. 2021).
Obesity stimulates the development of gene-induced hypoxia and adipogenesis in obese mice. Obesity increases the likelihood of developing immune-mediated and certain inflammatory-mediated illnesses, such as atherosclerosis and psoriasis, by dampening the immunological response to infectious pathogens, resulting in weakened post-infection effects. Furthermore, the obese host produces a unique milieu for disease development, characterized by low-grade inflammation that persists. To protect our bodies and reduce the danger of infectious illnesses, including COVID-19, it is recommended to maintain good eating habits by increasing the intake of diverse plant-based and low-fat meals (Behl et al. 2020).
The virus appears to enter the CNS mainly via the angiotensin-converting enzyme-2 (ACE-2) receptor and nasal route through the olfactory bulb and cribriform plate and propagates through trans-synaptic signaling, and moves retrogradely into the CNS along the nerve fiber, according to evidence. Parkinsonism, Alzheimer’s disease, meningitis, encephalopathy, anosmia, hyposmia, anxiety, sadness, psychiatric symptoms, seizures, stroke, and other problems have been linked to viral invasion of the CNS. As a consequence, even after the individual has cured from COVID-19, the COVID-19 CNS components should be checked on a regular basis to prevent long-term CNS issues (Nagu et al. 2020).
Diagnosis
Recently, two types of tests have been performed for the diagnosis:
-
a.
Molecular tests
-
b.
Antigen tests.
Molecular test detects viral genome whereas antigen tests target characteristic viral proteins.
Antibody test targets the antibodies that have been produced in patient’s blood with response to viral infection but it can be detected in post-COVID-19 patients also (Shahab et al. 2021; Ojha et al. 2021; Shoaib et al. 2021). Diagnostic tests are compiled in Table 3 and Fig. 4.
Drugs used in the management of SARS COV-2 infection
The repurposed drugs used in the management of SARS COV-2 infections (Pawelczyk and Zaprutko 2020; Parasher 2021; Saxena 2020; Gil et al. 2020) are listed and compiled in Table 4. Different drugs along with the mechanism of action in the management of SARS COV-2 are shown in Fig. 5.
Mechanism of action of drugs for the treatment of SARS-COV-2
Chloroquine/hydroxychloroquine
The mechanism of chloroquine and hydroxyl-chloroquine drugs involves the conversion of chloroquine to hydroxychloroquine which in turn inhibits several enzymes. Due to enzyme inhibition, viral entry is inhibited due to these weak bases because of pH dependency. The drug also inhibits glycosyl-transferases and post-translation modifications and also inhibits viral families. The mechanism of action is depicted in Fig.6 (Schrezenmeier and Dörne 2020).
Favipiravir
Favipiravir is an approved drug employed for severe influenza virus infection in China. It is available in an inactive form and gets converted into an active form by the action of enzymes into favipiravir ribo-furanosyl monophosphate (RMP) which further converts into the active form (favipiravir ribo-furanosyl triphosphate) which in turn can block the replication of several RNA viruses currently in clinical trials in COVID-19 management. The mechanism of action has been presented in Fig. 7 (Sood et al. 2021).
Remdesivir
It is a broad-spectrum antiviral drug; adenosine analog that can determine pre-mature termination of viral RNA. It has been tested for Ebola virus infection and might be useful in the treatment of other RNA virus infections. It has been shown that the action against SARS COV-2 infection also, still, studies are going on and showed in Fig. 8 (Beigel et al. 2019; Wang et al. 2020a).
Monoclonal antibodies
Monoclonal antibodies can be produced by immunizing the rats with antigens but sometimes failed to secrete antibodies. This can be further modified by mixing the myeloma cells with plasma cells from the spleen. Cell fusion results in hybridomas cells, which are transferred to hat medium and incubated. Hybridomas cells are further selected which produced antibodies known as monoclonal antibodies. These antibodies are found to be effective against COVID-19 and came in light when given to the ex-US president Donald Trump. The monoclonal antibody cocktail is a combination of two or more monoclonal antibodies which is administered to the patient as a single dose. These are found to exhibit activity by acting on the spike protein of the COVID-19 and do not allow it to enter into the human cell. For the treatment of mild to moderate covid infection, the combination of Bamlanivimab (700mg) and the mixture of Casirivimab and Imdevimab (2400mg) appeared to accelerate decline SARS-COV-2 level compared to placebo. Casirivimab and Imdevimab are mainly human immunoglobulin G-1 (IgG1). Figure 9 describes a method of production of monoclonal antibodies (Richardson et al. 2020; Ceribelli et al. 2020; Marovich et al. 2020; Zost et al. 2020; Goyal et al. 2021;Yang et al. 2020; Clinical Trial Arena 2020; Phan et al. 2021).
Recently, Zydus claimed regarding the repurposing of “Virafin” (pegylated interferon alpha-2b) in COVID-19 treatment which was earlier used in the treatment of the hepatitis C virus. Recently, Virafin has been given limited emergency approval from DCGI, India (Science The Wire 2021).
One more drug named “2-DG” has been developed by DRDO along with Dr. Reddy’s Lab. Hyderabad has been granted emergency approval from DCGI, India as an adjunct therapy to overcome oxygen demand. This drug has been developed in the treatment of cancer as it possesses promising cytotoxic potential (Economic times. Indiatimes 2021).
Currently, Canadian company Sanotize developed nitric oxide–based nasal spray, claimed to kill COVID-19 load inside the nose up to 99.99 %. Now, the company has filed for getting emergency approval in the UK and started its production in Israel (India today 2021).
Approved and phase 3 trial vaccines
Vaccines are the hope for controlling this pandemic caused by the COVID-19 virus. Few vaccines have been approved by regulatory authorization whereas some of them are in the process. It should be very clear that no vaccine is 100% effective until now; still results will prove the efficacy and safety of the vaccines. Each and every person must follow the guidelines and hygienic conditions. Scientists all over the world are working at war level for the production of vaccines; until now, 79 vaccines are still in process and among them, 11 vaccines have been approved by regulatory authorities whereas 20 vaccines are still in phase III clinical trials (Li and De Clercq 2020; Li et al, 2020a; Zoomer 2021; Graham, 2020) Vaccines that have been approved by regulatory authorities until now are shown in Table 5. The list of approved vaccines in phase trial 3 is compiled in Table 6.
Nano-medicines in SARS Cov-2 infection
Nano-technology possesses enormous potential in targeted drug delivery at the target site with minimum side effects, especially in the anticancer domain. This technology has been extensively utilized in the development of vaccines against the COVID-19 virus.
Viruses can be categorized as nanoparticles, which work at similar measures as other nanomaterials. There has been a lot of research performed as well as ongoing to mimic the nanoparticle-like virus behavior for designing the target drug release as well as gene delivery regimens (Singh et al. 2017). Therefore, nanotechnology may offer great value in the present ongoing pandemic via various ways such as viral neutralization and detection, vaccine developments, and providing effective treatment (Florindo et al. 2020).
Nanoparticles possess similarity to coronavirus, excluding viral genome if nanoparticles enter the host cells renovate immunity against this type of infection. Due to similar size nanoparticles may bind to the COVID-19 virus and distort its structure along with IR treatment which further results in hampering the viral survival and reproducibility(Yang 2021).
Various nanoparticles such as gold and carbon quantum dots (CQDs) are excellent options for interacting with the virus as well as averting entry within the cells due to the large surface area as well as a broad range of functional groups. CQDs possess a diameter 10 nm approx. and good water solubility, making it an ideal applicant for conquering coronavirus, due to easy entry through endocytosis and preventing mRNA replication (Wang et al. 2019; Iannazzo et al. 2018).
Gold nanoparticles using extracts impart additional advantages such as being eco-friendly, non-toxic, cost-effective, and easily accessible. Research performed so far revealed that gold nanoparticles can be stabilized with few polymers (bio-compatible) that might act as antiviral agents against HIV 1, FMDB, diarrhea, dengue, H1N1, H3N2, and H5N1 virus (Medhi et al. 2020). Silver, iron, mesoporous silica (Wang et al. 2017; Lara et al. 2010; Rojas et al. 2020; Rai et al. 2016) and organic particles such as Carbon nanotubes & Nanoparticles Graphene, Polymeric, Lipid-based, Dendrimers revolution in nanotechnology employing novel nanocarriers and magnetic nanoparticles (Comparetti et al 2018; Alidori et al. 2016; Mangum et al. 2006; Singh et al. 2019; Hennig et al. 2015; Figueroa et al. 2019; Pollock et al. 2010; Jain et al. 2011; Shah et al. 2017; Lembo et al. 2018; Li et al. 2005; Clayton et al. 2009; Itani et al. 2020; Williams & Corr 2013) have been documented in the treatment of Covid-19. The current strategies of nanoparticles used in the treatment of COVID-19 infection are shown in Table 7.
Discussion
An epidemic of pneumonia that started in December 2019 (Wuhan-China) has been well known to be due to novel coronavirus, which has been named later as COVID-19. The virus is extremely contagious and results in pandemic situations due to the lack of any medicine for prophylaxis as well as treatment.
Subsequently, searching the effective prophylactic and therapeutic strategies for the management of the disease is still continuing. Furthermore, the emergency requirement of drugs leads to the repurposing of drugs which has been mentioned in this article. We illustrated categorized response based on infection and contiguity stages of diseases among the human population. Despite the fact that until now no medicine system provides clinically proven treatment for COVID-19, continuous research is ongoing searching for the effective treatment for COVID-19(Fig. 10)(Silva et al. 2020; Sadlon et al. 2010; Rosa et al. 2020; Jin et al. 2020).
Moreover, vaccines, anti-viral drugs, and antibiotics possess the gold standard for the current preventive measures for different epidemic disorders until now. Furthermore, problems related to developing dispensing, side effects, storage conditions, mutations, and antibiotic-resistant microbes should also be taken into considerations. Novel approaches for communicable diseases are absolutely required and the WHO also mentioned that at the time of the Ebola outbreak in 2014. The expert committee stated that it’s unethical to suggest unproven intercession with unknown efficiency as well as adverse effects as promising treatment/prophylaxis. It should be taken into consideration that it’s not only COVID-19, but there are also other viruses as well as diseases for their vaccines, and clinically proven treatment is not available. Therefore, innovative research, as well as technique, is absolutely required for providing effective medication to society (Fig. 11)(Veronese et al. 2020; Reyes et al. 2020; Michael and Thompson 2020; Cox et al. 2021; Furuta et al. 2017; Cristian et al. 2020; Chaccour et al. 2021).
Challenges and issues observed during vaccine development
Vaccine development takes an average of 10–15 years, and compressing it to just 15 months has its own set of drawbacks and issues. Combining stages to accelerate vaccine development necessitates testing on smaller groups. This is a major issue because, if the vaccine is made available to the general public, unexpected adverse effects may develop in bigger groups that were previously undetected in smaller groups. Furthermore, if all individuals with comorbidities are not properly addressed in the design of clinical trials, unexpected adverse effects may be discovered in those groups once the vaccine is ready for general use. Vaccines would be examined for similar adverse effects in the broader population under post-marketing surveillance. It’s uncertain how mRNA vaccines will be developed since in past researches. Nucleic acid–based vaccines like DNA and RNA have failed to generate viable vaccinations for human diseases in the past. Because lipid nanoparticles are temperature sensitive, scaling up production may be difficult. Pre-existing adenovirus immunity is a concern, particularly for vaccine candidates that utilize human adenoviruses, such as CanSino’s Ad5 vaccine, since it may reduce the vaccine’s immunological response (Sharma et al. 2020).
In order to meet pandemic demand, rapid large-scale vaccine production remains a challenge fraught with uncertainties. Because phase 3 trials need more than 30,000 individuals and are performed later in the study phase, there is a high chance that there will be fewer instances of COVID-19 at that time, requiring HCTs. Despite the fact that HCTs have been utilized in the past, they may pose a higher risk for COVID-19 due to a lack of understanding of the disease's genesis and the absence of a feasible treatment. As an alternative to HCTs, several vaccine candidates have taken advantage of transmission rate differences by starting phase 3 trials in countries with a higher SARS-CoV-2 infection rate to ensure that a significant number of patients can participate. Vaccines against the virus may be ineffective due to the virus’s mutations. However, given the urgent need for a COVID-19 vaccine to be accessible globally, being concerned about and analyzing such risks should not prevent the public introduction of otherwise safe and effective vaccines (Sharpe et al. 2020, Morris 2020, Sharma et al. 2020).
Future perspective
The COVID-19 pandemic is still a global health issue that has impacted a vast number of people. From December 2019 onwards, most nations around the world have recorded a substantial number of COVID-19 cases. There are currently no effective vaccinations or medications available to prevent COVID-19 infection. To address these health issues, various research groups are working to determine the most effective treatment for COVID-19 by delivering FDA-approved antiviral medicines such as oseltamivir, ritonavir, remdesivir, ribavirin, and favipiravir, among others. Simultaneously, scientists are concentrating on the creation of several types of vaccinations that can prevent COVID-19 infection. According to the survey results, research will continue until acceptable drug candidates and vaccines are found based on pathological conditions, physiology, clinical symptoms, diagnosis, and public health emergencies.
Conclusion
Keeping in view of this epidemic, we’re still waiting for precise COVID-19 treatment and care. Although a few medicines, such as remdesivir, ribavirin, and favipiravir, are prescribed for the therapy, it is difficult to employ them specifically for the treatment of COVID-19 infection due to a lack of clinical data. These medicines were discovered to have a high affinity for the COVID-19 primary protease. Antimalarial medications like chloroquine and hydroxychloroquine, on the other hand, had a strong binding affinity with the SARS spike glycoprotein and the ACE2 complex. Vaccination has begun in India, although clinical data on safety and efficacy will not be available for some time. Even after immunization, everyone should be upbeat and follow the instructions to the letter. It’s also been noted that we’re dealing with not only COVID-19 but also its fear, particularly in India.
Data Availability
Available on request.
Abbreviations
- ACE:
-
angiotensin-converting enzyme
- ANGPTL3:
-
angiopoietin-like protein 3
- COVID-19:
-
corona virus disease-19
- FDA:
-
Food and Drug Administration
- FPV:
-
Favipiravir
- HIV:
-
human immunodeficiency virus
- MERS-CoV:
-
Middle East Respiratory Syndrome-Coronavirus
- MOA:
-
mechanism of action
- Mpro:
-
main protease
- RDV:
-
remdesivir
- RdRp:
-
RNA-dependent RNA polymerase
- RNA:
-
ribonucleic acid
- SARS-CoV-2:
-
severe acute respiratory syndromecoronavirus-2
- TCZ:
-
tocilizumab
- WHO:
-
World Health Organization
References
Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR, Gallagher T, Enjuanes L (2018) Coronavirus Susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio 9. https://doi.org/10.1128/mBio.00221-18
Akerstrom S, Mousavi-Jazi M, Klingstom J et al (2005) Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J Virol. https://doi.org/10.1128/JVI.79.3.1966-1969.2005
Alhazzani W, Evans L, Alshamsi F, Møller MH, Ostermann M, Prescott HC, Arabi YM, Loeb M, Ng Gong M, Fan E, Oczkowski S, Levy MM, Derde L, Dzierba A, du B, Machado F, Wunsch H, Crowther M, Cecconi M et al (2021) Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 49:e219–e234. https://doi.org/10.1097/CCM.0000000000004899
Alidori S, Bowman RL, Yarilin D, Romin Y, Barlas A, Mulvey JJ, Fujisawa S, Xu K, Ruggiero A, Riabov V, Thorek DLJ, Ulmert HDS, Brea EJ, Behling K, Kzhyshkowska J, Manova-Todorova K, Scheinberg DA, McDevitt MR (2016) Deconvoluting hepatic processing of carbon nanotubes. Nat. Commun. 7:12343. https://doi.org/10.1038/ncomms12343
Aouba A, Baldolli A, Geffray L et al (2020) Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. https://doi.org/10.1136/annrheumdis-2020-2177069
Arabi YM, Fowler R, Hayden FG (2020) Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 46:315–328. https://doi.org/10.1007/s00134-020-05943-5
Bauer SR, Kapoor A, Rath M, Thomas SA (2020) What is the role of supplementation with ascorbic acid, zinc, vitamin D, or N-acetylcysteine for prevention or treatment of COVID-19? Cleve Clin J Med. https://doi.org/10.3949/ccjm.87a.ccc046
Behl T, Kaur I, Bungau S, Kumar A, Uddin MS, Kumar C, Pal G, Sahil SK, Zengin G, Arora S (2020) The dual impact of ACE2 in COVID-19 and ironical actions in geriatrics and pediatrics with possible therapeutic solutions. Life Sci. 257:118075. https://doi.org/10.1016/j.lfs.2020.118075
Beigel JH, Nam HH, Adams PL, Krafft A, Ince WL, el-Kamary SS, Sims AC (2019) Advances in respiratory virus therapeutics - a meeting report from the 6th isirv antiviral group conference. Antiviral Res. 167:45–67. https://doi.org/10.1016/j.antiviral.2019.04.006
Bermejo-Martin JF, Kelvin DJ, Eiros JM et al (2009) Macrolides for the treatment of severe respiratory illness caused by novel H1N1 swine influenza viral strains. J Infect Dev Ctries. https://doi.org/10.3855/jidc.18
Bloch EM, Bailey JA, Tobian AAR (2020) Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. https://doi.org/10.1172/JCI138745
Bozkurt B, Kovacs R, Harrington B (2020) Joint HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19. J Card Fail. https://doi.org/10.1016/j.cardfail.2020.04.013
Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM (2020) The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 178:104787. https://doi.org/10.1016/j.antiviral.2020.104787
Carfora V, Spiniello G, Ricciolino R, di Mauro M, Migliaccio MG, Mottola FF, Verde N, Coppola N, Vanvitelli COVID-19 group, Coppola N, Sagnelli C, de Pascalis S, Stanzione M, Stornaiuolo G, Cascone A, Martini S, Macera M, Monari C, Calò F et al (2021) Anticoagulant treatment in COVID-19: a narrative review. J Thromb Thrombolysis. 51:642–648. https://doi.org/10.1007/s11239-020-02242-0
Ceribelli A, Motta F, De Santis M (2020) Recommendations for coronavirus infection in rheumatic diseases treated with biologic therapy. J Autoimmun. https://doi.org/10.1016/j.jaut.2020.102442
Cevik M, Kuppalli K, Kindrachuk J, Peiris M (2020) Virology, transmission, and pathogenesis of SARS-CoV-2. BMJ. https://doi.org/10.1136/bmj.m3862
Chaccour C, Casellas A, Matteo AB, Pineda I, Montero AF, Castillo PR et al (2021) The effect of early treatment with Ivermectin on viral load, symptoms and humoral response in patients with non-severe COVID-19: a pilot, double-blind, placebo-controlled, randomized clinical trial. E-Clinical Medicine. 32:100720. https://doi.org/10.1016/j.eclinm.2020.100720
Chen L, Liu P, Gao H, Sun B, Chao D, Wang F, Zhu Y, Hedenstierna G, Wang CG (2004) Inhalation of nitric oxide in the treatment of severely acute respiratory syndrome: a rescue trial in Beijing. Clin Infect Dis. 39:1531–1535. https://doi.org/10.1086/425357
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J', Yu T, Zhang X, Zhang L (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan. China: a descriptive study. Lancet. 395:507–513. https://doi.org/10.1016/S0140-6736(20)30211-7
Cherian SV, Kumar A, Akasapu K (2018) Salvage therapies for refractory hypoxemia in ARDS. Respir Med. 141:150–158. https://doi.org/10.1016/j.rmed.2018.06.030
Chu CM, Cheng VC, Hung IF, Wong MM, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY, HKU/UCH SARS Study Group (2004) Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 59:252–256. https://doi.org/10.1136/thorax.2003.012658
Clayton R, Ohagen A, Nicol F, Del Vecchio AM, Jonckers THM, Goethals O et al (2009) Sustained and specific in vitro inhibition of HIV-1 replication by a protease inhibitor encapsulated in gp120-targeted liposomes. Antiviral Res. 84:142–149. https://doi.org/10.1016/j.antiviral.2009.08.003
Clinical Trial Arena (2020) Bamlanivimab (LY-Cov555) for the Treatment of Covid-19. https://www.clinicaltrialsarena.com/projects/bamlanivimab-ly-cov555-for-the-treatment-of-covid-19/. Clinicaltrialsarena Web. Accessed 04 May 2021.
Comparetti EJ, de Pedrosa VA, Kaneno R (2018) Carbon nanotube as a tool for fighting cancer. Bioconjug. Chem. 29:709–718. https://doi.org/10.1021/acs.bioconjchem.7b00563
Covid-19 Vaccine tracker (2021). Approved Vaccines. https://covid19.trackvaccines.org/vaccines/approved/. Accessed on 22 July 2021.
Cox RM, Wolf JD, Plemper RK (2021) Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nature Microbiology 6(1) 11-18 10.1038/s41564-020-00835-2
Cristian F, Lucie F, Boris B, Timo W, Elisabeth HA (2020) Severe COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Mult Scler Relat Disord 42:102180. DOI:10.1016/j.msard.2020.102180
Deftereos SG, Siasos G, Giannopoulos G, Vrachatis DA, Angelidis C, Giotaki SG, Gargalianos P, Giamarellou H, Gogos C, Daikos G, Lazanas M, Lagiou P, Saroglou G, Sipsas N, Tsiodras S, Chatzigeorgiou D, Moussas N, Kotanidou A, Koulouris N et al (2020) The GReek study in the Effects of Colchicine in COvid-19 complications prevention (GRECCO-19 study): rationale and study design. Hellenic J Cardiol. 61:42–45. https://doi.org/10.1016/j.hjc.2020.03.002
Del Rio C, Malani PN (2020) 2019 Novel Coronavirus—Important Information for Clinicians. JAMA 323(11) 1039-10.1001/jama.2020.1490
Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, Hong Z, Xia J (2020a) Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: a retrospective cohort study. J Infect. 81:e1–e5. https://doi.org/10.1016/j.jinf.2020.03.002
Deng Y, Liu W, Liu K (2020b) Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan. China: a retrospective study. Chin Med J (Engl). 133:1261–1267. https://doi.org/10.1097/CM9.0000000000000824
Dong L, Hu S, Gao J (2020a) Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug DiscovTher. 14:58–60. https://doi.org/10.5582/ddt.2020.01012
Dong S, Sun J, Mao Z, Wang L, Lu YL, Li J (2020b) A guideline for homology modeling of the proteins from newly discovered betacoronavirus, 2019 novel coronavirus (2019-nCoV). J Med Virol. 92:1542–1548. https://doi.org/10.1002/jmv.25768
Economictimes.indiatimes (2021) What is 2-deoxy-D-glucose(2-DG) and is it effective against Covid?economictimes.indiatimes Web. https://economictimes.indiatimes.com/industry/healthcare/biotech/pharmaceuticals/what-is-2-deoxy-d-glucose-2-dg-and-is-it-effective-against covid/articleshow/82567938.cms?utm_ source = content of interest &utm_medium=text&utm_campaign=cppst. Accessed on 22 August 2021.
Fang L, Karakiulakis G, Roth M (2020) Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 8:e21. https://doi.org/10.1016/S22132600(20)30116-8
Favalli EG, Biggioggero M, Maioli G, Caporali R (2020) Baricitinib for COVID-19: a suitable treatment. Lancet Infect Dis. 20:1012–1013. https://doi.org/10.1016/S1473-3099(20)30262-0
Fda.gov (2021) Emergency Use Authorization. Fda.gov Web. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs. Accessed on 22 July 2021.
Figueroa SM, Veser A, Abstiens K, Fleischmann D, Beck S, Goepferich A (2019) Influenza A virus mimetic nanoparticles trigger selective cell uptake. Proc. Natl Acad Sci U.S.A. https://doi.org/10.1073/pnas.1902563116
Florindo HF, Kleiner R, Vaskovich-Koubi D, Acúrcio RC, Carreira B, Yeini E, Tiram G, Liubomirski Y, Satchi-Fainaro R (2020)Immune-mediated approaches against COVID-19. Nat. Nanotechnol. 15:630–645. https://doi.org/10.1038/s41565-020-0732-3
Frost FJ, Petersen H, Tollestrup K, Skipper B (2007) Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest. 131:1006–1012. https://doi.org/10.1378/chest.06-1997
Furuta Y, Komeno T, Nakamura T (2017) Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc JpnAcad Ser B Phys Biol Sci. 93:449–463. https://doi.org/10.2183/pjab.93.027
Gandolfini I, Delsante M, Fiaccadori E, Zaza G, Manenti L, Degli Antoni A, Peruzzi L, Riella LV, Cravedi P, Maggiore U (2020)COVID-19 in kidney transplant recipients. Am J Transplant. 20:1941–1943. https://doi.org/10.1111/ajt.15891
Ghosh S, Das S, Mondal R, Abdullah S, Sultana S, Singh S, Sehgal A, Behl T (2021) A review on the effect of COVID-19 in type 2 asthma and its management. Int Immunopharmacol. 91:107309. https://doi.org/10.1016/j.intimp.2020.107309
Gil C, Ginex T, Maestro I, Nozal V, Barrado-Gil L, Cuesta-Geijo MÁ, Urquiza J, Ramírez D, Alonso C, Campillo NE, Martinez A (2020) COVID-19: drug targets and potential treatments. J Med Chem. 63:12359–12386. https://doi.org/10.1021/acs.jmedchem.0c00606
Goyal M, Tewatia N, Vashisht JR, Kumar S (2021) Novel corona virus (COVID-19). Global efforts and effective investigational medicines: a review. J Infect Public Health. 14:910–921. https://doi.org/10.1016/j.jiph.2021.04.011
Graham BS (2020) Rapid COVID-19 vaccine development. Science. 368:945–946. https://doi.org/10.1126/science.abb8923
Griffiths MJD, McAuley DF, Perkins GD et al (2019) Guidelines on the management of acute respiratory distress syndrome. BMJ Open Resp Res. 6:e000420. https://doi.org/10.1136/bmjresp-2019-000420
Hashimoto K (2021) Repurposing of CNS drugs to treat COVID-19 infection: targeting the sigma-1 receptor. Eur Arch Psychiatry Clin Neurosci. 271:249–258. https://doi.org/10.1007/s00406-020-01231-x
Hennig R, Veser A, Kirchhof S, Goepferich A (2015) Branched polymer– drug conjugates for multivalent blockade of angiotensin II receptors. Mol Pharm. 12:3292–3302. https://doi.org/10.1021/acs.molpharmaceut.5b00301
Huang Y, Yang C, Xu X, Wei Xu W, Liu S (2020) Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 41:1141–1149. https://doi.org/10.1038/s41401-020-0485-4
Huet T, Beaussier H, Voisin O et al (2020) Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020. 10.1016/S2665- 9913(20)30164-8
Iannazzo D, Pistone A, Ferro S, De Luca L, Monforte AM, Romeo R et al (2018) Graphene quantum dotsbased systems as HIV inhibitors. Bioconjug. Chem. https://doi.org/10.1021/acs.bioconjchem.8b00448
Indiatoday (2021) Covid-19: SaNOtize files for emergency approval in UK, Canada for its nasal spray treatment. Indiatoday Web. https://www.indiatoday.in/coronavirus-outbreak/story/covid-19-sanotize-files-for-emergency-approval-in-uk-canada-for-its-nasal-spray-treatment-1794286-2021-04-23.
Intrado GlobeNewswire (2020) FDA resumes eIND approval for severe-to-criticalCOVID-19 patients use of Vyrologix™ (leronlimab) following full enrollment in CytoDyn’s Phase 3 Trial. Globenewswire Web. https://www.globenewswire.com/news-release/2020/12/22/2149221/0/en/FDA-Resumes-eIND-Approval-for-Severe-to-Critical-COVID-19-Patients-Use-of-Vyrologix-leronlimab-Following-Full-Enrollment-in-CytoDyn-s-Phase-3-Trial.html. Accessed 04 May 2021.
Itani R, Tobaiqy M, Al Faraj A (2020) Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics. 10:5932–5942. https://doi.org/10.7150/thno.46691
Jain S, Mistry MA, Swarnakar NK (2011) Enhanced dermal delivery of acyclovir using solid lipid nanoparticles. Drug Deliv. Transl. Res. 1:395–406. https://doi.org/10.1007/s13346-011-0036-0
Jain U (2020) Effect of COVID-19 on the Organs. Cureus. https://doi.org/10.7759/cureus.9540
Jawhara S (2020) Could intravenous immunoglobulin collected from recovered coronavirus patients protect against COVID-19 and strengthen the immune system of new patients? Int J Mol Sci. 21. https://doi.org/10.3390/ijms21072272
Jena NR (2021) Drug targets, mechanisms of drug action, and therapeutics against SARS-CoV-2. Chemical Physics Impact. https://doi.org/10.1016/j.chphi.2021.100011
Jha NK, Jeyaraman M, Rachamalla M, Ojha S, Dua K, Chellappan D, Muthu S, Sharma A, Jha S, Jain R, Jeyaraman N, GS P, Satyam R, Khan F, Pandey P, Verma N, Singh S, Roychoudhury S, Dholpuria S et al (2021) Current understanding of novel coronavirus: molecular pathogenesis, diagnosis, and treatment approaches. Immuno. 1:30–66. https://doi.org/10.3390/immuno1010004
Jin YH, Zhan QY, Peng ZY, Ren XQ, Yin XT (2020) Chemoprophylaxis, diagnosis, treatments, and discharge management of COVID-19: an evidence-based clinical practice guideline (updated version). Mil Med Res. 7:41. https://doi.org/10.1186/s40779-020-00270-8
Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C (2010) Mode of antiviral action of silver nanoparticles against HIV-1. J Nano-biotechnol. 8:1. https://doi.org/10.1186/1477-3155-8-1
Lembo D, Donalisio M, Civra A, Argenziano M, Cavalli R (2018) Nanomedicine formulations for the delivery of antiviral drugs: a promising solution for the treatment of viral infections. Expert Opin Drug Deliv. 15:93–114. https://doi.org/10.1080/17425247.2017.1360863
Li F, Li W, Farzan M, Harrison SC (2005) Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 309:1864–1868. https://doi.org/10.1126/science.1116480
Li G, De Clercq E (2020) Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov. 19:149–150. https://doi.org/10.1038/d41573-020-00016-0
Li T, Lu H, Zhang W (2020a) Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 9:687–690. https://doi.org/10.1080/22221751.2020.1741327
Li Y, Xia L (2019) Coronavirus disease (COVID-19): role of chest CT in diagnosis and management. Cardiovasc Imaging. https://doi.org/10.2214/AJR.20.22954, Coronavirus Disease 2019 (COVID-19): Role of Chest CT in Diagnosis and Management
Li YD, Chi WY, Su JH, Ferrall L, Hung CF, Wu TC (2020b) Coronavirus vaccine development: from SARS and MERS to COVID-19. J Biomed Sci. 27:104. https://doi.org/10.1186/s12929-020-00695-2
LianN XH, LinS HJ, ZhaoJ LQ (2020) Umifenovir treatment is not associated with improved outcomes in patients with Corona-virus disease 2019: a retrospective study. Clin Microbiol Infect. 26:917–921. https://doi.org/10.1016/j.cmi.2020.04.026
Lou Y, Liu L, Yao H, Hu X, Su J, Xu K, Luo R, Yang X, He L, Lu X, Zhao Q, Liang T, Qiu Y (2021) Clinical outcomes and plasma concentrations of baloxavirmarboxil and favipiravir in COVID-19 patients: an exploratory randomized, controlled trial. Eur J Pharm Sci. 157:105631. https://doi.org/10.1016/j.ejps.2020.105631
Lu H (2020) Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 14:69–71. https://doi.org/10.5582/bst.2020.01020
Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J (2020) Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 92:814–818. https://doi.org/10.1002/jmv.25801
Mangum JB, TurpinEA A-MA, Cesta MF, Bermudez E, Bonner JC (2006)Single-walled carbon nanotube (SWCNT)- induced interstitial fibrosis in the lungs of rats is associated with increased levels of PDGF mRNA and the formation of unique intercellular carbon structures that bridge alveolar macrophages in situ. Part. Fibre Toxicol. 3. https://doi.org/10.1186/1743-8977-3-15
Mantlo E, Bukreyeva N, Maruyama J et al (2020) Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. https://doi.org/10.1016/j.antiviral.2020.104811
Markham A, Keam SJ (2018) Danoprevir: first global approval. Drugs. 78:1271–1276. https://doi.org/10.1007/s40265-018-0960-0
Marovich M, Mascola JR, Cohen MS (2020) Monoclonal antibodies for prevention and treatment of COVID-19. JAMA. 324:131–132. https://doi.org/10.1001/jama.2020.10245
Mastrangelo E, Pezzullo M, De Burghgraeve T et al (2012) Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: new prospects for an old drug. J Antimicrob Chemother. https://doi.org/10.1093/jac/dks147
McCarty MF, DiNicolantonio JJ (2020) Nutraceuticals have potential for boosting the type 1 interferon response to RNA viruses including influenza and coronavirus. Prog Cardiovasc Dis. https://doi.org/10.1016/j.pcad.2020.02.007
Medhi R, Srinoi P, Ngo N, Tran HV, Lee TR (2020)Nanoparticle-based strategies to combat COVID-19. ACS Appl Nano Mater. 3:8557–8580. https://doi.org/10.1021/acsanm.0c01978
Michael AM, Thompson BT (2020) Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties. Lancet. 8:1170–1172. https://doi.org/10.1016/S2213-2600(20)30503-8
Moore HB, Barrett CD, Moore EE, McIntyre RC, Moore PK, Talmor DS, Moore FA, Yaffe MB (2020) Is there a role for tissue plasminogen activator (tPA) as a novel treatment for refractory COVID-19 associated acute respiratory distress syndrome (ARDS)? J Trauma Acute Care Surg. 88:713–714. https://doi.org/10.1097/TA.0000000000002694
Morris KV (2020) The improbability of the rapid development of a vaccine for SARS-CoV-2. Mol Ther. 28:1548–1549. https://doi.org/10.1016/j.ymthe.2020.06.005
Myron SC (2021) Monoclonal antibodies to disrupt progression of early Covid-19 infection. N Engl J Med.10.1056/NEJMe2034495
Nagu P, Parashar A, Behl T, Mehta V (2020) CNS implications of COVID-19: a comprehensive review. Rev Neurosci. 32:219–234. https://doi.org/10.1515/revneuro-2020-0070
National Health Commission and State Administration of Traditional Chinese Medicine (2020) Diagnosis and treatment protocol for novel coronavirus pneumonia. 5 August 2020.
Nguyen NNT, McCarthy C, Lantigua D, Camci-Unal G (2020) Development of Diagnostic Tests for Detection of SARS-CoV-2. Diagnostics (Basel). 10. https://doi.org/10.3390/diagnostics10110905
Ojha PK, Kar S, Krishna JG, Roy K, Leszczynski J (2021) Therapeutics for COVID-19: from computation to practices—where we are, where we are heading to. Mol Diver. 25:625–659. https://doi.org/10.1007/s11030-020-10134-x
Parasher A (2021) COVID-19: current understanding of its pathophysiology, clinical presentation and treatment. Postgrad Med J. 97:312–320. https://doi.org/10.1136/postgradmedj-2020-138577
Pawelczyk A, Zaprutko L (2020)Anti-COVID drugs: repurposing existing drugs or search for new complex entities, strategies and perspectives. Future Med Chem. 12:1743–1757. https://doi.org/10.4155/fmc-2020-0204
Perrella A, Carannante N, Berretta M, Rinaldi M, Maturo N, Rinaldi L (2020) Novel Coronavirus 2019 (Sars-CoV2): a global emergency that needs new approaches? Eur Rev Med Pharmacol. 10.26355/eurrev_202002_20396
Phadke M, Saunik S (2020)COVID-19 treatment by repurposing drugs until the vaccine is in sight. Drug Dev Res. 81:541–543. https://doi.org/10.1002/ddr.21666
Phan AT, Gukasyan J, Arabian S et al (2021) Emergent inpatient administration of Casirivimab and Imdevimab antibody cocktail for the treatment of COVID-19 pneumonia. Cureus. https://doi.org/10.7759/cureus.15280
Pineda B, Perez V, Pandoo RH, Sotelo J (2021) Quinacrine as a potential treatment for COVID-19 virus infection. Eur Rev Med PharmacolSci. 10.26355/eurrev_202101_24428
Pollock S, Branza NN, Böhmer A, Radulescu C, Dwek RA, Zitzmann N (2010) Polyunsaturated liposomes are antiviral against hepatitis B and C viruses and HIV by decreasing cholesterol levels in infected cells. Proc Natl Acad Sci. 107:17176–17181. https://doi.org/10.1073/pnas.1009445107
Pottoo FH, Izneid TA, Ibrahim AM et al (2021) Immune system response during viral Infections: Immunomodulators, cytokine storm (CS) and Immunotherapeutic in COVID-19. Saudi Pharm J. https://doi.org/10.1016/j.jsps.2020.12.018
Rai M, Deshmukh SD, Ingle AP, Gupta IR, Galdiero M, Galdiero S (2016) Metal nanoparticles: the protective nanoshield against virus infection. Crit. Rev. Microbiol. 42:46–56. https://doi.org/10.3109/1040841X.2013.879849
RECOVERY Collaborative Group (2020)Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 396:1345–1352. https://doi.org/10.1016/S0140-6736(20)32013-4
Regulatory Focus (2021). COVID-19 vaccine tracker. RAPS Web. https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker. Accessed on Accessed on 22 July 2021.
Reyes AZ, Hu KA, Teperman J et al (2020)Anti-inflammatory therapy for COVID-19 infection: the case for colchicines. Ann Rheum Dis Epub. https://doi.org/10.1136/annrheumdis-2020-219174
Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, Rawling M, Savory E, Stebbing J (2020) Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 395:e30–e31. https://doi.org/10.1016/S0140-6736(20
Rojas M, Rodríguez Y, Monsalve DM, Acosta-Ampudia Y, Camacho B, Gallo JE et al (2020) Convalescent plasma in Covid-19: possible mechanisms of action. Autoimmun. Rev. 19:102554.10.1016/j.autrev.2020.102554
Rosa SGV, Santos WC (2020) Clinical trials on drug repositioning for COVID-19 treatment. Rev PanamSalud Publica 20(44):e40. https://doi.org/10.26633/RPSP.2020.40
Rosen DA, Seki SM, Fernández-Castañeda A, Beiter RM, Eccles JD, Woodfolk JA, Gaultier A (2019) Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci Transl Med. 11:eaau5266. https://doi.org/10.1126/scitranslmed.aau5266
Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan China. Intensive Care Medicine 46(5) 846-848. https://doi.org/10.1007/s00134-020-05991-x
Russell CD, Millar JE, Baillie JK (2020) Clinical evidence does not support corticosteroid treatment for 2019-CoV lung injury. Lancet. 395:473–475
Sadlon AE, Lamson DW (2010)Immune-modifying and antimicrobial effects of eucalyptus oil and simple inhalation devices. Altern Med Rev J Clin Ther.
Salama C, Han J, Linda Y, William G, Reiss KB (2021) Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 384:20–30. https://doi.org/10.1056/NEJMoa2030340
Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N (2020) Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 178:104791. https://doi.org/10.1016/j.antiviral.2020.104791
Sanders JM, Monogue ML, Jodlowski et al (2020) Pharmacologic treatments for coronavirus diseases 2019 (COVID-19): a review. JAMA. https://doi.org/10.1001/jama.2020.6019
Saxena A (2020) Drug targets for COVID-19 therapeutics: Ongoing global efforts. J Biosci. 45. https://doi.org/10.1007/s12038-020-00067-w
Schrezenmeier E, Dörne T (2020) Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Rheumatology. 23:82–91. https://doi.org/10.1016/s0049-0172(10)80012-5
Science The Wire (2021). DCGI approves virafin for moderate COVID. where’s the evidence it works.https://science.thewire.in/the-sciences/zydus-virafin-pegylated-interferon-alpha-2b-india-dcgi-approve-covid-trial-methods-flaw. Science.thewire Web. Accessed 04 May 2021.
Shah MR, Imran M, Ullah S (2017)Lipid-Based Nanocarriers for Drug Delivery and Diagnosis. https://doi.org/10.1016/B978-0-323-52729-3.00009-3
Shahab MS, Imam SS, Jahangir MA (2021) A review on the contemporary status of mutating coronavirus and comparative literature study of current COVID-19 vaccines. Int J Pharm Pharmacol.:10.31531/2581-3080.1000153
Sharma O, Sultan AA, Ding H, Triggle CR (2020) A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front Immunol. 11. https://doi.org/10.3389/fimmu.2020.585354
Sharpe HR, Gilbride C, Allen E, Belij‐Rammerstorfer S, Bissett C, Ewer K, Lambe T (2020) The early landscape of coronavirus disease 2019 vaccine development in the UK and rest of the world. Immunology 160(3) 223-232 10.1111/imm.13222
Shoaib MH, Ahmed FR, Sikandar M, Yousuf RI, Saleem MT (2021) A journey from SARS-CoV-2 to COVID-19 and beyond: a comprehensive insight of epidemiology, diagnosis, pathogenesis, and overview of the progress into its therapeutic management. Front Pharmacol. 12. https://doi.org/10.3389/fphar.2021.576448
Siemieniuk RA, Meade MO, Alonso-Coello P et al (2015) Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 163:519–528. https://doi.org/10.7326/M15-0715
Silva J, Figueiredo P, Byler K, Setzer W (2020) Essential oils as antiviral agents. Potential of essential oils to treat SARS-CoV-2 infection: an in-silico investigation. Int J Mol Sci 21. https://doi.org/10.3390/ijms21103426
Simonds AK, Hanak A, Chatwin M, Morrell MJ, Hall A, Parker KH, Siggers JH, Dickinson RJ (2010) Evaluation of droplet dispersion during non-invasive ventilation, oxygen therapy, nebuliser treatment and chest physiotherapy in clinical practice: implications for management of pandemic influenza and other airborne infections. Health Technol Assess. 14:131–172. https://doi.org/10.3310/hta14460-02
Singh AP, Biswas A, Shukla A, Maiti P (2019) Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct Target Ther. https://doi.org/10.1038/s41392-019-0068-3
Singh L, Kruger HG, Maguire GEM, Govender T, Parboosing R (2017) The role of nanotechnology in the treatment of viral infections. The role of nanotechnology in the treatment of viral infections. Ther Adv Infect Dis. 4:1177/2049936117713593–1177/2049936117713131
Sood S, Bhatia GK, Seth P, Kumar P, Kaur J, Gupta V, Punia S, Tuli HS (2021) Efficacy and safety of new and emerging drugs for COVID-19: Favipiravir and Dexamethasone. Current PharmacolRep. 7:49–54. https://doi.org/10.1007/s40495-021-00253-w
Tiberghien P, De Lambalarie X, Morel P et al (2020) Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how. VOX. 115:488–494. https://doi.org/10.1111/vox.12926
Tran DH, Sugamata R, Hirose T, Suzuki S, Noguchi Y, Sugawara A, Ito F, Yamamoto T, Kawachi S, Akagawa KS, Ōmura S, Sunazuka T, Ito N, Mimaki M, Suzuki K (2019) Azithromycin, a 15-membered macrolide antibiotic, inhibits influenza A (H1N1)pdm09 virus infection by interfering with virus internalization process. J Antibiot (Tokyo). 72:759–768. https://doi.org/10.1038/s41429-019-0204-x
U.S. National Library of Medicine (2020)ClinicalTrials.gov. Accessed 2020 Jul 19. https://clinicaltrials.gov/ct2/show/study/NCT04370782. Accessed 21 August 2020.
U.S. National Library of Medicine (2021) Clinical Trials.gov. https://clinicaltrials.gov/ct2/show/NCT04337359. Accessed 12 Feb 2021.
Veronese N, Demurtas J, Yang L, Tonelli R, Barbagallo M, Lopalco P, Lagolio E, Celotto S, Pizzol D, Zou L, Tully MA, Ilie PC, Trott M, López-Sánchez GF, Smith L (2020) Use of corticosteroids in coronavirus disease 2019 pneumonia: a systematic review of the literature. Front Med 7. https://doi.org/10.3389/fmed.2020.00170
Wang C, Zhu W, Wang BZ (2017)Dual-linker gold nanoparticles as adjuvanting carriers for multivalent display of recombinant influenza hemagglutinin trimers and flagellin improve the immunological responses in vivo and in vitro. Int J Nanomed. https://doi.org/10.2147/IJN.S137222
Wang M, Cao R, Zhang L et al (2020a) Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. https://doi.org/10.1038/s41422-020-0282-0
Wang X, Feng Y, Dong P, Huang J (2019) A mini review on carbon quantum dots: preparation, properties, and electrocatalytic application. Front Chem 7. https://doi.org/10.3389/fchem.2019.00671
Wang Y, Fei D, Vanderlaan M, Song A (2004) Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. https://doi.org/10.1007/s10456-004-8272-2
Wang Y, Jiang W, He Q, Wang C, Wang B, Zhou P et al (2020b) A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther.10.1038/s41392-020-0158-2
WHO (2021)https://extranet.who.int/Web.https://extranet.who.int/pqweb/sites/default/files/documents/StatusCOVIDVAX23April2021.pdf. Accessed on 22 July 2021.
Williams MJ, Corr SA (2013) Magnetic nanoparticles for targeted cancer diagnosis and therapy. Front Nanosci. https://doi.org/10.1016/B978-0-08-098338-7.00002-9
Wu C, Liu Y, Yang Y et al (2020) Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. https://doi.org/10.1016/j.apsb.2020.02.008
Wu CJ, Jan JT, Chen CM, Hsieh HP, Hwang DR, Liu HW, Liu CY, Huang HW, Chen SC, Hong CF, Lin RK, Chao YS, Hsu JTA (2004) Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob Agents Chemother. 48:2693–2696. https://doi.org/10.1128/AAC.48.7.2693-2696.2004
Xu J, Shi PY, Li H, Zhou J (2020) Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect Dis. 6:909–915. https://doi.org/10.1021/acsinfectdis.0c00052
Yan ZP, Yang M, Lai CL (2021)COVID-19 vaccines: a review of the safety and efficacy of current clinical trials. Pharmaceuticals. 14. https://doi.org/10.3390/ph14050406
Yang B, Fulcher JA, Ahn J, Berro M, Goodman-Meza D, Dhody K, Sacha JB, Naeim A, Yang OO (2020) Clinical characteristics and outcomes of coronavirus disease 2019 patients who received compassionate-use leronlimab. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1583
Yang D (2021) Application of Nanotechnology in the COVID-19 Pandemic. Int J Nanomedicine. Volume 16:623–649. https://doi.org/10.2147/IJN.S296383
Zhang C, Wu Z, Li JW, Zhao H, Wang GQ (2020a) The cytokine release syndrome (CRS) of severe COVID-19 and interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. https://doi.org/10.1016/j.ijantimicag.2020.105954
Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, Zeng X, Zhang S (2020b) The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the perspectives of clinical immunologists from China. Clin Immunol. 214:108393. https://doi.org/10.1016/j.clim.2020.108393
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579:270–273. https://doi.org/10.1038/s41586-020-2012-7
Zoomer (2021). COVID-19 Update: NACI weighs in on “preferred” vaccines; mixing doses is possibility. https://www.everythingzoomer.com/health/2021/05/04/covid-19-what-we-know-about-the-second-wave-and-the-targeted-approach-for-protecting-canadians. Everythingzoomer Web. Accessed 04 May 2021.
Zost SJ, Gilchuk P, Case JB et al (2020) Potently neutralizing and protective human antibodies against SARS-CoV-2. Nature. https://doi.org/10.1038/s41586-020-2548-6
Acknowledgements
The authors acknowledge the Management of Devsthali Vidyapeeth College of Pharmacy for providing continuous support for the article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization V.T. and A.T.; writing—original draft preparation M.K. and B.M.S.; writing—review and editing S.S., S.K., and R.S. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Lotfi Aleya
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tiwari, V., Kumar, M., Tiwari, A. et al. Current trends in diagnosis and treatment strategies of COVID-19 infection. Environ Sci Pollut Res 28, 64987–65013 (2021). https://doi.org/10.1007/s11356-021-16715-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-16715-z