Abstract
After months of restrictive containment efforts to fight the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) epidemic, European countries are planning to reopen. To support the process, we conducted a cross-sectional survey among the Hungarian population to estimate the prevalence of infectious cases and prior SARS-CoV-2 exposure. A representative sample (n = 17,787) for the Hungarian population of 14 years or older living in private households (n = 8,283,810) was selected. The study was performed within 16 days after 50 days of restrictions, when the number of confirmed cases was stable low. Naso- and oropharyngeal smears and blood samples were collected for PCR and antibody testing. The testing was accompanied by a questionnaire about symptoms, comorbidities, and contacts. Design-based prevalence estimates were calculated. In total, 10,474 individuals (67.7% taken into account a sample frame error of 2315) of the selected sample participated in the survey. Of the tested individuals, 3 had positive PCR and 69 had positive serological test. Population estimate of the number of SARS-CoV-2 infection and seropositivity were 2421 and 56,439, respectively, thus active infection rate (2.9/10,000) and the prevalence of prior SARS-CoV-2 exposure (68/10,000) was low. Self-reported loss of smell or taste and body aches were significantly more frequent among those with SARS-CoV-2. In this representative, cross-sectional survey of the Hungarian population with a high participation rate, the overall active infection rate was low in sync with the prevalence of prior SARS-CoV-2 exposure. We demonstrated a potential success of containment efforts, supporting an exit strategy. NCT04370067, 30.04.2020.
Similar content being viewed by others
Introduction
With the outbreak of the corona virus disease 2019 (COVID-19) pandemic, efforts to estimate the total number infections and to investigate the prior exposure to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) are key elements of developing defensive responses. (WHO announces COVID-19 outbreak a pandemic 2020; Guan et al. 2020) International health organizations endorsed general recommendations of strategic preparedness and outlined preventive measures as a part of a response plan in support of all countries. (Coronavirus Disease 2019 (COVID-19) 2020a; Country and Technical Guidance—Coronavirus disease (COVID-19) 2020) Even though these recommendations were incorporated almost universally, each country has experienced a distinct course of the epidemic depending on the timing of safety measure initiation and the degree of compliance with the restrictive measures.
In Europe, the first cluster of cases were confirmed on February 21 in Lombardy, Italy (Onder et al. 2020) (~ 450 miles away from Hungary). As of March 13, Europe was declared to be the active center of the COVID-19 pandemic by the World Health Organization. (Coronavirus disease 2019 (COVID-19) 2020b)
In Hungary, the first two SARS-CoV-2 cases were diagnosed on March 4 (university students who had returned from Asia). By March 11, altogether 16 laboratory-confirmed infections as well as 1 COVID-19–related death were registered. At that time, a state emergency was declared and universities were closed. Subsequently from March 16, further restrictions were introduced, such as prohibition of public gatherings of more than 100 people, closing elementary and high schools, reducing the opening hours of restaurants and cafes, as well as permitting the entry to Hungary of citizens only. Subsequently, on March 28, general lockdown was announced, public events were canceled, and only grocery stores and pharmacies were allowed to remain open; at the time 343 confirmed cases were registered and 11 SARS-CoV-2–related death occurred. Some of the safety measures concentrated only on the most vulnerable segment of the population, the elderly. One example is selective opening hours, providing a period of the day, when the grocery stores, pharmacies, and markets were only open for individuals aged 65 or older.
After around 6 weeks of lockdown, a slow opening of the economy was initiated within countries across Europe. However, in order to safely and effectively execute such a complex process, estimating the total number of infective cases and the prevalence of previous exposure to the pathogen is essential. Investigation of the SARS-CoV-2 virulence and case severity has been extensively studied among patients with severe disease course. (Guan et al. 2020; Onder et al. 2020) However, in order to reveal the full spectrum of disease and adjust public health safety measures accordingly, the rate of mild or asymptomatic infections that do not require medical attention need to be explored. (Lipsitch et al. 2020)
The objective of the present study was to estimate the total number of infectious cases and the prevalence of prior SARS-CoV-2 exposure in the Hungarian population after 50 days of strict containment measures. The study was conducted to support the development of an exit policy from the currently applied safety restrictions.
Methods
Study design, patient population
The study was funded by the 2020-2.1.1-ED-2020-00017 grant and was approved by the institutional review board and the local ethics committee (IRB IV/4060-3/2020/EKU).
The target population included individuals aged 14 years or older, living in private households in Hungary. A two-stage stratified probability sample of individuals was selected from the population registry, selecting settlements as primary sampling units (PSU) at the first stage and individuals at the second stage. To obtain equal precision in each region, seven regional samples of equal size were designed. Within each region, the larger settlements as well as settlements with at least five confirmed cases became certainty PSUs. The 181 certainty PSUs cover 55% of the target population and 82% of the overall number of confirmed cases. Within each region, settlements with one to four confirmed cases constituted separate strata and the rest of PSUs were stratified by size, taxable income per capita, and population with tertiary educational level. Overall, 154 strata were defined this way and two PSUs were selected with probability proportional to size within each strata. Altogether, 489 settlements from 3155 were selected. Within the settlements, individuals were selected by systematic random sampling after ordering them by age, as age had been identified as the single most important factor associated with the severity of the infection. A minimum of four individuals were selected from each selected settlement. The total number of the sample size was determined by assuming 10% sampling frame error and 70% participation rate. Thus, 17,787 individuals were selected to ensure a planned effective sample size of 11,206. To ensure the temporally cross-sectional nature of the study, the data collection period was restricted to 16 days that fell under the same restriction regulations starting on May 1.
Those participants were included in the final study population who had either PCR test or serological testing with a completed questionnaire (Fig. 1).
Contacting selected individuals
We informed selected individuals with an official invitation letter via mail. Those who had previously authorized to be contacted officially by e-mail received an additional invitation electronically. In addition, 14,250 participants with a telephone number registered in the administrative databases were contacted by phone by the Central Statistical Office and the universities. Almost 3000 general practitioners and the local municipalities provided help to contact and motivate the selected participants. Finally, those who we failed to be contacted were visited on their registered address.
Screening process and sample collection
Screening was carried out as a collaborative effort of four medical universities across Hungary (Semmelweis University, University of Debrecen, University of Szeged, University of Pécs) and the Hungarian Central Statistical Office. Individuals selected for screening were required to register online or over the phone through a dedicated line. The samples were collected by 187 screening teams in 348 fixed screening posts, in five screening buses, and by mobile testing units through a personal visit to those who could not be mobilized. These efforts were supported by the ambulance service, municipalities, and local governmental offices.
Laboratory measurements
PCR test
To detect or exclude the presence of SARS-CoV-2 virus, nasopharyngeal and oropharyngeal samples were collected in viral transport medium tubes and were transported to the laboratory at 2–8 °C. After nucleic acid extraction, real-time PCR was performed (HBRT-COVID-19; Chaozhou Hybribio Biochemistry Ltd., Chaozhou, Guangdong, China) (Organization WH 2020). PCR was performed within 24 h after sample collection. The test used detects the presence of two SARS-CoV-2 viral genes and also applies a human gene sequence as an internal quality control for sampling. The absence of amplified human gene in PCR product indicates inappropriate sampling. Patients with inappropriate samples were re-sampled the day after and provided samples that were appropriate for testing.
Those who tested positive were informed within 2 days and were required to self-isolate for 2 weeks. Positive test results were also reported toward the Hungarian Health Authorities. Participants had access to their own results via the National eHealth Infrastructure.
Blood sample analysis
For serological testing, blood samples were obtained from all participants at the age of 18 or older; under 18 years of age, blood testing was optional. Serological testing for SARS-CoV-2–specific IgG was analyzed with commercially available Food and Drug Administration–approved immunoassay (SARS-CoV-2 IgG Reagent Kit Cat. No. 6R86-32 on Architect i2000SR instruments; Abbott Laboratories, Irving, TX, USA), (Abbott 2020) and the remnant samples were stored at − 80 °C.
Questionnaire
All participants were invited to fill out a questionnaire during the registration process online, over the phone, or during the screening in person. The questionnaire contained questions regarding socio-economic status, risk factors (smoking and body mass index—BMI), comorbidities, adherence to safety measures, recent travel history, history of symptoms suggestive for COVID-19, and history of a known contact with a confirmed SARS-CoV-2–infected individual or with a person in quarantine.
Definition of confirmed cases
To provide a comprehensive background of the COVID-19 epidemic in Hungary, beyond the results of this current survey, we report the age, sex, and regional distribution of all reported confirmed cases and COVID-19–related mortality registered in Hungary until May 16 according to the National Public Health Center. We provide this data for individuals at the age of 14 years or older, living in private households and separately for those living in institutions (homeless shelter, long-term care facility or nursery home).
Statistical analysis
We estimated the population prevalence of acute infection and seropositivity by age, sex, and region, categories of labor activity, contact with a person infected with SARS-CoV-2 or being in quarantine, and by visiting a foreign country since March 1, 2020.
To reduce bias in weighting, we could use several area-, dwelling unit-, and individual-level auxiliary information from sampling frame and other administrative data sources, each related to both non-response and the objective variable. After adjusting design weights, the response sample was calibrated to known population counts by region, sex, and age categories.
Variance estimation method took calibration effect as well as stratification and clustering into account. For the residuals by calibration variables, we used Taylor-linearized variance estimation. (Wolter 2007) In case of zero observation in a subgroup, we used the rule-of-three method to estimate confidence intervals (i.e., 95% confidence interval of the prevalence was estimated as 0–3/n). (Eypasch et al. 1995) The calculations were performed using SAS software version 9.4.
Results
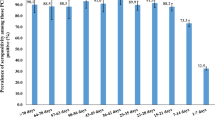
Of the planned calculated sample of 17,787 individuals, 10,505 underwent PCR testing and 10,504 had a serological test, while 10,434 had both. In total, 12,236 individuals completed the questionnaire. Altogether, 10,474 people, 67.7% of the 15,472 individuals belonging to the sampling frame, who had either a PCR or a serological test with a completed questionnaire, were included into our final study population (Fig. 1). The mean age was 48.7 years and the 46.4% were male (Table 1). The population estimate of the proportion of individuals who experienced any symptoms suggestive for SARS-CoV-2 infection was larger among those with a positive PCR or immunological test compared with seronegative persons: 54.7% vs. 42.2% (Fig. 2c). Body aches and loss of smell or taste occurred significantly more frequently among people with a positive test (19.0%, 95% CI 8.6–29.3% vs. 7.8%, 95% CI 7.2–8.4%, and 14.1%, 95% CI 5.8–22.4 vs. 2.6%, 95% CI 2.3–2.9%, respectively). Shortness of breath and diarrhea was also a common symptom in the seropositive group, although compared with the seronegatives, the difference was not statistically significant. The estimated proportion in the population of people who had any comorbidities was higher among persons with a positive test: 54.4% vs. 41.5% (Fig. 2d).
Test results in the studied population
Three participants had a positive PCR test; two of them were hospitalized due to confirmed COVID-19 infection. Altogether, 70 individuals had a positive serological test result, 69 with a completed questionnaire. Of the seropositive individuals, two had a simultaneous positive PCR test.
Estimated population infection rate
Based on the results of the survey, the estimated number of PCR-positive and seropositive individuals in the entire Hungarian population 14 years or older who live in independent households (n = 8,283,810) were 2421 and 56,439, respectively.
The estimates in Table 2 suggest that seropositivity tends to increase by age (between 14 and 39 years—56/10,000 vs. 65 years or older—83/10,000) and is higher among those who commuted regularly to their workplace (commutes several times—59/10,000 vs. worked from home—42/10,000), had a contact with confirmed SARS-CoV-2–infected individual or someone in quarantine (individuals with a history of contact—114/10,000 vs. without a contact—66/10,000), and traveled abroad after March 12, 2020 (traveled abroad—106/10,000 vs. no travel—67/10,000). However, none of these differences were statistically significant.
By large statistical region, the highest prevalence of seropositivity was found in the central region including the capital, Budapest (Fig. 3). By subregion, the difference was larger between Budapest (90/10,000) and the two least developed regions: Southern Transdanubia (46/10,000) and Northern Hungary (45/10,000) (Table 3).
The total number of the reported confirmed cases by the National Public Health Center in Hungary until the end of the study was 3464, of whom 2580 lived in private households and were 14 years or older. In the latter group, the number of confirmed new cases during the study period of May 1–16 ranged between 15 and 56, an average 27 per day. The number of registered deaths was 350 and 101 among persons living in private households and in institutions, respectively. The age distribution of the confirmed cases reported until May 16 living in private households was similar to the age distribution of the test-positive subjects identified in the survey (Table 4). The regional variation was larger in the confirmed cases than in seropositive ones: among those who live in private households, 61% of the confirmed cases occurred in the Central region, whereas seropositive individuals were distributed evenly by large statistical region (Tables 3 and 4). The sex and regional distributions of the confirmed cases were identical among those who lived in private households and in institutions. On the other hand, the proportion of elderly people was twice as large in the latter group than in the former.
Discussion
In the H-UNCOVER representative, cross-sectional population survey of Hungarian individuals, we investigated the total number of active infections and prevalence of virus exposure after 50 days of the initiation of containment regulations. We provide the first comprehensive analysis utilizing PCR and serological testing simultaneously. Of the selected representative population sample, 67.7% was tested and completed the questionnaire resulting in a considerably high participation rate. Despite the close proximity of major infectious clusters, due to the early introduction of containment efforts, the overall active infection rate remained low (2.9/10,000), in sync with the prevalence of SARS-CoV-2 exposure (68/10,000).
The relatively higher rate of participation in comparison with similar studies conducted to describe the epidemic of SARS-CoV-2 (Gudbjartsson et al. 2020) could be attributed to multiple factors. First, in order to mobilize as many participants as possible, we contacted individuals on three parallel levels: each person was notified via mail and over the phone; as well as general practitioners from around the country contacted selected individuals from their practice. Second, investigators of our study were supported by local governors and the media to spread information to reach out to as many individuals as possible. Third, to enhance the accessibility of testing units, we established even distribution of designated testing facilities and mobile testing units across the country. In addition, we provided in-person visits for two reasons: to further improve the study participation and to test individuals who were otherwise not mobilizable. Lastly, the availability of participants in their homes was considerably higher due to the quarantine and travel restrictions.
In this survey, a relatively low active SARS-CoV-2 infection rate (0.029%) and in sync low overall seropositive rate was identified (0.68%). In Hungary, 12 days after the first SARS-CoV-2–infected cases were confirmed, restrictive measures were implemented. The authors believe that the early initiation of strict containment efforts may explain the control of the spread of SARS-CoV-2 infection. It is important to underline that there were also specific safety measures in Hungary solely applied to elderly people, such as specific opening hours when only the elderly population could visit markets, grocery stores, or pharmacies. In addition, the adherence of the Hungarian population to these regulations also has to be emphasized. Data from different sources including online surveys, depersonalized aggregated mobile cell and traffic data showed a drastic, 60–90% reduction in the number of contacts and extreme reduction in mobility. (Röst et al. 2020) It has been shown with the use of mobility and COVID-19 epidemiology data from the European countries that successful restrictive policy can significantly contribute to the suppression of the SARS-CoV-2 pandemic. (Vokó and Pitter 2020)
Furthermore, consistently with the low level of seropositivity reported in our study, the rate of incident cases registered by the National Public Health Center in Hungary throughout the timeframe of the testing was low as well. However, besides the early containment efforts, there have been a few additional potential protective factors suggested against COVID-19, such as BCG vaccination or blood type 0 which is representative in the Hungarian population. (Curtis et al. 2020; Zhao et al. 2020) In Hungary, BCG vaccination is mandatory, and type 0 is relatively frequent, thus the population may have lower susceptibility to SARS-CoV-2; however, more robust evidence needs to be provided to prove the protective effect of these factors.
The proportion of reported confirmed cases was substantially higher in the Central region of Hungary than the proportion of seropositivity as identified via the cross-sectional survey, which can be explained by the higher rate of testing performed in this area: among those with a PCR test performed until May 16, 43% was obtained in the Central region, which represents 30% of the total population.
Our results suggest a higher SARS-CoV-2 infection rate in older individuals and with persons with chronic diseases, which have been shown to be a risk factor for more severe disease course. (Grasselli et al. 2020) We must also note that a large proportion of reported cases occurred in institutions (Table 4), mainly in nursing homes. Among them 74% were 65 years or older, and mortality was much higher among elderly people. (Kemenesi et al. 2020) The reason for this might be that in older individuals, the immune response is less effective. Moreover, there is evidence that in older individuals suffering from SARS-CoV-1 infection, the switch from innate to adaptive immunity is impaired resulting in an insufficient antibody production, which seems to be the case in SARS-CoV-2 infection as well. (Nikolich-Zugich et al. 2020) This highlights the need for specific interventions and safety measures in elderly care facilities as part of the exit strategy, such as prolonged restrictive measures including strict visitor policy, rigorous cleaning and disinfection protocols, mandatory use of personal protective equipment by the staff, appropriate training of the personnel, viral testing of the new residents, frequent surveillance of symptoms suggestive for SARS-CoV-2, and adequate policies and organization to isolate individuals with suspected infection.
In line with prior evidence, our data also showed that loss of smell or taste might be associated with SARS-CoV-2 infection. (Spinato et al. 2020) We also found that diarrhea was more common among seropositive people, which is in accordance with the previously reported Italian and Chinese data. (Lin et al. 2020; Buscarini et al. 2020)
Based on the results reported in the present study exploring the epidemiology of SARS-CoV-2 exposure, the development of an exit strategy from the currently applied containment regulations is feasible. General regulations considered to be essential to control the spread of SARS-CoV-2, which were introduced all over the world, were adapted in Hungary in a relatively early phase. Due to differences in the implementation of safety recommendations, however, each country may have its own characteristic course of the epidemic. Therefore, we believe that each country needs to survey their population in order to learn their unique characteristic of COVID-19 epidemic and to develop their individual strategic plan to establish a transition back to normal everyday living and to resuscitate the economy. We acknowledge that a sensitive balance between people’s health and economy exists; however, an early opening of the economy might undermine positive effects of restrictive efforts. Our study suggests that early initiation of safety measures and adherence to regulations can decrease the spread of SARS-CoV-2 and result in low COVID-19–related morbidity and mortality, especially protecting the elderly (aged 65 and older) who are the most vulnerable against the SARS-CoV-2 infection.
Results of the present study mirror the situation after a time period of 50 days of safety restrictions. In order to track the effect of economic reopening and loosening of restrictive safety measures, repeating this representative population-based cross-sectional survey with a nested longitudinal follow-up of a subgroup is planned. Moreover, as future waves of COVID-19 are predicted, until herd immunity is not obtained or until large-scale vaccination cannot be maintained, the need for such cross-sectional surveys are even more pronounced.
Our survey had certain limitations. First, selective non-response could be considered as a potential limitation; however, it is very unlikely that the participation in the study was related to a previously undiagnosed SARS-CoV-2 infection. Second, while PCR testing is regarded as the current standard diagnostic method for SARS-CoV-2 infection, the risk of false negativity can be significant and could be decreased significantly with repeated sampling which was not feasible in such a study.
In conclusion, our study suggests that early initiation of containment efforts and adherence to regulations may decrease the spread of SARS-CoV-2 and could result in low COVID-19–related morbidity and mortality. Consequently, an exit strategy from the currently applied containment regulations is feasible.
References
Abbott Receives FDA Emergency Use Authorization For Covid-19 Antibody Blood Test On Alinity™ I System. https://abbott.mediaroom.com/2020-05-11-Abbott-Receives-FDA-Emergency-Use-Authorization-for-COVID-19-Antibody-Blood-Test-on-Alinity-TM-i-System (2020). Accessed 05.13.2020.
Buscarini E, Manfredi G, Brambilla G, Menozzi F, Londoni C, Alicante S, et al. GI symptoms as early signs of COVID-19 in hospitalised Italian patients. Gut. 2020:gutjnl-2020-321434. https://doi.org/10.1136/gutjnl-2020-321434.
Coronavirus Disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/communication/guidance-list.html?Sort=Date%3A%3Adesc (2020a). Accessed 05.13. 2020.
Coronavirus disease 2019 (COVID-19) https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200313-sitrep-53-covid-19.pdf?sfvrsn=adb3f72_2 (2020b). Accessed 05.13. 2020.
Country & Technical Guidance—Coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance (2020). Accessed 05.13. 2020.
Curtis N, Sparrow A, Ghebreyesus TA, Netea MG. Considering BCG vaccination to reduce the impact of COVID-19. Lancet. 2020;395(10236):1545–6. https://doi.org/10.1016/S0140-6736(20)31025-4.
Eypasch E, Lefering R, Kum CK, Troidl H. Probability of adverse events that have not yet occurred: a statistical reminder. BMJ. 1995;311(7005):619–20. https://doi.org/10.1136/bmj.311.7005.619.
Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574. https://doi.org/10.1001/jama.2020.5394.
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. https://doi.org/10.1056/NEJMoa2002032.
Gudbjartsson DF, Helgason A, Jonsson H, Magnusson OT, Melsted P, Norddahl GL, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382:2302–15. https://doi.org/10.1056/NEJMoa2006100.
Kemenesi G, Kornya L, Tóth GE, Kurucz K, Zeghbib S, Somogyi BA, et al. Nursing homes and the elderly regarding the COVID-19 pandemic: situation report from Hungary. Geroscience. 2020:1–7. https://doi.org/10.1007/s11357-020-00195-z.
Lin L, Jiang X, Zhang Z, Huang S, Fang Z, Gu Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69(6):997–1001. https://doi.org/10.1136/gutjnl-2020-321013.
Lipsitch M, Swerdlow DL, Finelli L. Defining the epidemiology of Covid-19—studies needed. N Engl J Med. 2020;382(13):1194–6. https://doi.org/10.1056/NEJMp2002125.
Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience. 2020;42(2):505–14. https://doi.org/10.1007/s11357-020-00186-0.
Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020. https://doi.org/10.1001/jama.2020.4683.
Organization WH: SARS-CoV-2 nucleic acid tests: progress of the active applications in the emergency use listing assessment pipeline. https://www.who.int/diagnostics_laboratory/200514_eul_covid19_ivd_update.pdf (2020). Accessed 05.13. 2020.
Röst G, Bartha FA, Bogya N, Boldog P, Dénes A, Ferenci T, et al. Early phase of the COVID-19 outbreak in Hungary and post-lockdown scenarios. Submitted to Viruses 2020.
Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323:2089. https://doi.org/10.1001/jama.2020.6771.
Vokó Z, Pitter JG. The effect of social distance measures on COVID-19 epidemics in Europe: an interrupted time series analysis. Geroscience. 2020:1–8. https://doi.org/10.1007/s11357-020-00205-0.
WHO announces COVID-19 outbreak a pandemic. http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic (2020). Accessed 05.13.2020.
Wolter MK. Introduction to variance estimation. New York: Springer; 2007. p. 354–66.
Zhao J, Yang Y, Huang H, Li D, Gu D, Lu X, et al. Relationship between the ABO blood group and the COVID-19 susceptibility. medRxiv. 2020.
Acknowledgments
We thank Gergely Fraller for his contribution to the data analysis, the National Ambulance Service, the municipalities of the settlements involved in the survey, and to the staff of the governmental offices for their valuable help in the survey. We greatly appreciate the permission of the National Public Health Center to present the summary data of the confirmed cases.
Funding
Open access funding provided by Semmelweis University. The study was funded by the 2020-2.1.1-ED-2020-00017 grant of National Research Development and Innovation Office of Hungary. The study sponsor had no role in the study design, writing, or interpretation of the data, and had no role in the decision to submit the article for publication. The researchers were independent from funders and all authors (external and internal) had full access to all data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Consortia
Contributions
The concept of the study was developed by B.M. and was designed by B.M., A.K., G.M., J.K., K.M., and Z.V. The sampling and the analysis was planned by K.M. and Z.V. The data collection was organized and led by B.M., C.L., E.B., A.M., A.J.S., K.B., I.C., A.S., I.V., C.P., J.B., P.V., and G.Á.F. Laboratory tests were organized by B.V. and B.H. Data were analyzed by K.M. Infectology advice was provided by E.L., G.P. and J.S. The first version of the manuscript was drafted by B.M., A.K., G.M., J.K., K.M., and Z.V. M.B. acts as guarantor. All authors contributed to the interpretation of the results and to the revision of the subsequent versions of the manuscript, and approved the final version of the manuscript. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The corresponding author attests that all listed authors meet authorship criteria and that there were no others who met the criteria.
All listed investigators of H-UNCOVER (HUNgarian COronaVirus-19 Epidemiological Research) helped to organize and/or conduct the screening at their local universities. The following are investigators of H-UNCOVER: József Gajdácsi, Katalin Kristóf, László Tamás, Álmos Gogl, Anita Czira, Kriszta Katinka Boros (Semmelweis University), Donát Drexler, Imre Földesi, Zoltán Pető, Viktória Sümegi, Balázs Bende (University of Szeged), József Kónya MD, János Kappelmayer, Bhattoa Harjit Pal, Zoltán Bács, Ákos Pintér (University of Debrecen), Imre Boncz, Ferenc Jakab, Katalin Gombos, Tamás Nagy, Antal Tibold (University of Pécs), Beatrix Oroszi (National Center for Public Health).
Corresponding author
Ethics declarations
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: as reported in the funding section, the current research was funded by the 2020-2.1.1-ED-2020-00017 grant of National Research Development and Innovation Office; otherwise, no financial relationships with any organizations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work were reported.
Ethical approval and consent to participate
The study protocol, the questionnaire, the screening procedures, and informed consent forms were approved by the institutional review board and the local ethics committee (IRB IV/4060-3/2020/EKU). All participants gave informed consent before taking part. Although the consent form is in Hungarian, upon request we are happy to provide an example.
Data sharing
We are happy to make the complete de-identified patient data set and the code for analysis/statistics available upon reasonable request.
Transparency statement
The manuscript’s guarantor (M.B.) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Consent to publish
I Béla Merkely, MD, PhD, the Corresponding Author of this article contained within the original manuscript which includes also diagrams & tables & figures submitted, has the right to grant on behalf of all authors and does grant on behalf of all authors a license to the Geroscience Journal, to permit this contribution (if accepted) to be published in the journal.
I am one author signing on behalf of all co-owners of the Contribution.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A complete list of investigators in the HUNgarian COronaVirus-19 Epidemiological Research (H-UNCOVER) is provided in the Declarations author contributions part.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Merkely, B., Szabó, A.J., Kosztin, A. et al. Novel coronavirus epidemic in the Hungarian population, a cross-sectional nationwide survey to support the exit policy in Hungary. GeroScience 42, 1063–1074 (2020). https://doi.org/10.1007/s11357-020-00226-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-020-00226-9