Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the outbreak of coronavirus disease 2019 (COVID-19). The COVID-19 numbers are continuously evolving worldwide (about 4 million confirmed cases and 260,000 deaths as of 9th May 2020), and Italy has registered a huge number of COVID-19 cases and deaths as compared to other countries [1]. Lung and systemic inflammation are the most common features of COVID-19, which may lead to multi-organ dysfunction and death [2]. Hence, extensive research in multiple therapeutic areas is rapidly progressing [3, 4]
Airborne particulate matter (PM), also called particle pollution, is considered to be the most relevant component of air pollution [5]. PM is a heterogeneous mixture of solid and liquid, organic and inorganic material suspended in air. The characteristics and sources of ultrafine PM (PM0.1, < 0.1 μm), fine PM (PM2.5, < 2.5 μm) and coarse PM (PM10, < 10 μm) are described elsewhere [5]. Numerous studies have documented the detrimental impact of PM air pollution on several organs [6], particularly airways and lungs [7, 8], although the quality of evidence remains poor in some cases [9]. The existence of a relationship between air pollution, including that from PM, and airborne viral infections has been also proposed [10]. In this regard, an association between air pollution, mainly PM10, and SARS mortality has been reported [11, 12]. In addition, exposure to ambient PM2.5 explained a significant percentage of incident cases of influenza [13] and measles in China [14]. Recently, a comprehensive association study found an independent link between six air pollutants, including PM2.5 and PM10, and the number of COVID-19 confirmed cases in 120 Chinese cities [15]. Also, a significant relationship between long-term exposure to nitrogen dioxide and COVID-19 fatalities has been described [16].
Several mechanisms have been proposed to explain the detrimental association between PM and viral infections: modifications of the respiratory immune response to microbial pathogens [10, 17], increased airways vulnerability to respiratory infections [18], exacerbation of multiple comorbidities [19], and additional under-researched mechanisms (e.g. increased viral spread due to attachment of viral particles to PM condensation nuclei) [20].
Based on these premises, on the excess of PM pollution in specific geographical areas in Italy [21], and on the recent debate on the excess of COVID-19 cases and deaths in some areas of Northern Italy as compared to the rest of the country [21–23], we tested the association between PM exposure and COVID-19 cases and deaths in different Italian regions and provinces.
Material and methods
Data collection
Data were extracted from publicly accessible databases and stratified by 20 Italian regions and up to 110 provinces (according to availability). The numbers of COVID-19 cases and deaths since 1st March to 31st March 2020 were retrieved from the daily bulletins of the Italian Ministry of Health (available at http://www.salute.gov.it). Data on the population size on 1st January 2019, the geographical extension of Italian regions and provinces, gender distribution, and the percentage of people aged ≥ 65 years were collected from the Italian National Statistical Institute (ISTAT) database (available at https://www.istat.it). Mean population exposure to PM2.5 and PM10 and the number of days exceeding the limit value of exposure to PM10 (50 µg/m3) in 2017 were extracted from the Italian National Institute for Environmental Protection and Research (ISPRA) databases (available at http://www.isprambiente.gov.it). The mean annual temperatures recorded in 2017 in different Italian provinces were extracted from the ISTAT database, and the mean relative humidity in the last three decades was sourced from an official Italian weather forecast website (www.ilmeteo.it).
Statistical analysis
SPSS statistical package, release 17.0 (SPSS Inc., Chicago, III) was used for statistical analyses. Base 10 logarithmic (LG) transformation was performed for skewed variables. The COVID-19 incidence proportion was calculated as the ratio between COVID-19 cases recorded in March 2020 and the regional-provincial population sizes per 100,000 inhabitants. The COVID-19-specific death rate was calculated as the ratio between COVID-19-related deaths recorded in March 2020 and the regional population sizes per 100,000 inhabitants (data for provincial death rates were unavailable). ANOVA was used to compare PM measurements across provinces by geographical distribution (North, Centre, South-Islands). Spearman’s and Pearson’s correlations were used to test the crude associations between the study variables. Multiple linear regression analyses were performed to test whether the exposure to PM2.5 and PM10 was associated with either COVID-19 incidence proportions across Italian regions and provinces or COVID-19-specific death rates across Italian regions. Variables with a significant crude association with COVID-19 endpoints (incidence proportion and cause-specific death rate) were included as covariates in the multivariable regression analyses; thus, gender distribution across regions and provinces, and territorial extension, were always included as independent confounders. In addition to the aforementioned covariates, mean annual temperature and relative humidity were included in the multivariable regression analyses carried out at the provincial level.
Results
COVID-19 cases and deaths
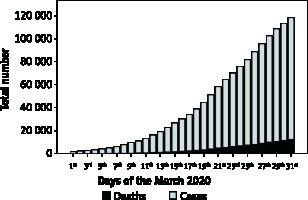
Figure 1 shows the Italian trend of COVID-19 cases and deaths since 1st March to 31st March 2020. An uneven distribution of population size, territorial extension, and number of COVID-19 cases and deaths were recorded across Italian regions up to 31st March 2020 (Table I). Lombardia was the Italian region with highest number of COVID-19 cases and deaths, accounting for 41% and 58% of the Italian COVID-19 cases and deaths, respectively. The lowest numbers of COVID-19 cases and deaths were recorded in Molise and Basilicata, respectively (Table I). Data on the number of COVID-19 cases stratified by date and Italian provinces are available at www.epicentro.iss.it.
Table I
Demographic characteristics and COVID-19 cases and deaths by geographical region
Exposure to PM2,5 and PM10 across Italian regions and provinces
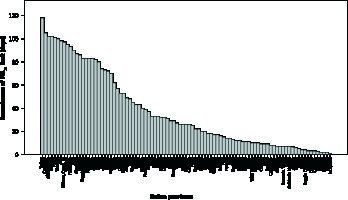
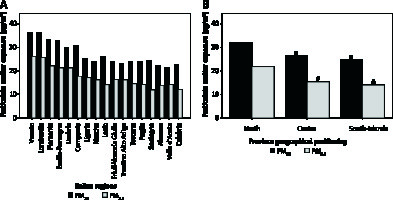
As depicted in Figure 2 A, Lombardia and Veneto were the Italian regions with the highest mean exposure to PM2.5 and PM10, whereas Sardegna and Valle d’Aosta were those with the lowest mean exposure to PM2.5 and PM10, respectively. Figure 2 B shows mean PM2.5 and PM10 exposure across Italian provinces stratified according to geographical positioning (i.e. North, Centre, South-Islands). Additional data regarding the number of days exceeding the limit value of exposure to PM10 (50 µg/m3) in 2017 are reported in Figure 3.
Figure 2.
Mean exposure to particulate matter (PM2.5 and PM10) in Italian regions (A) and provinces stratified according to geographical positioning (i.e. North, Centre, South-Islands) (B). ISPRA PM exposure data were not available for Basilicata and Molise regions, whereas public availability was limited to PM10 data for 6 provinces in the Sicilia region. *P < 0.001 for PM10 comparisons with Northern provinces, #p < 0.001 for PM2.5 comparisons with Northern provinces
Determinants of COVID-19 incidence proportions and death rate across Italian regions
Two multiple linear regression models were elaborated including the LG-COVID-19 incidence proportion as the dependent variable and the following independent variables: gender distribution, territorial region extension, and the regional mean annual exposure to PM2.5 and PM10 (Table II). Both PM2.5 and PM10 were associated with COVID-19 incidence proportion irrespective of confounders (β = 0.71, p = 0.003 and β = 0.61, p = 0.031, respectively). Two additional multiple linear regression models were elaborated including the LG-COVID-19 death rate as the dependent variable and the same independent variables as in the previous regression model (Table II). Again, both indices of PM exposure (i.e. PM2.5 and PM10) were independently associated with COVID-19 death rates across Italian regions (β = 0.68, p = 0.004 and β = 0.61, p = 0.029, respectively).
Determinants of COVID-19 incidence proportions across Italian provinces
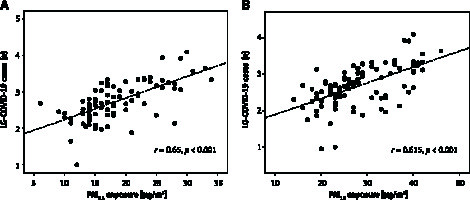
Crude positive correlations between the mean annual exposure to either PM2.5 (available complete data for 81 provinces) or PM10 (available complete data for 97 provinces), and LG-COVID-19 cases are depicted in Figures 4 A and B, respectively.
Figure 4.
Correlation between the number of COVID-19 cases in Italian provinces and either PM2.5 (A) or PM10 (B) exposure
Two multiple linear regression models were elaborated including the LG-COVID-19 incidence proportion and the following independent variables: gender distribution, territorial provincial extension, mean annual temperature, relative humidity, and the provincial mean annual exposure to either PM2.5 or PM10 (Table II). Both PM2.5 and PM10 exposures were independently associated with COVID-19 incidence proportion across Italian provinces (β = 0.26, p = 0.024 and β = 0.27, p = 0.006, respectively) (Table II). In the regression model with PM2.5 as an independent variable, both mean annual temperature (β = –0.46, p < 0.001) and relative humidity (β = 0.25, p = 0.024) were independently associated with COVID-19 incidence proportion. Similar results for annual temperature (β = –0.51, p < 0.001) and relative humidity (β = 0.23, p = 0.021) were observed in the PM10 multivariate model.
Table II
Adjusted associations between ISPRA-released data on PM2.5 and PM10 exposure and COVID-19 incidence proportion and death ra
An additional multiple linear regression analysis in Italian provinces was performed with LG-COVID-19 incidence proportion as the dependent variable and the number of days exceeding the limit value of exposure to PM10 (50 µg/m3) as an independent variable along with confounders. Also in this regression model, the index of PM10 exposure was independently associated with COVID-19 incidence proportion (β = 0.30, p = 0.008) (Table II).
Discussion
In this study, exploring the association between past PM2.5 and PM10 and the excess of COVID-19 related features (i.e. cases and deaths) in Italy, two main results emerged. First, an extremely heterogeneous distribution of COVID-19 cases and deaths across Italian regions and provinces was evident. Second, region-stratified and province-stratified exposure to PM2.5 and PM10 was associated with COVID-19 incidence proportion and cause-specific death rate, irrespective of demographic and climatic confounders.
In the analysis restricted to the Italian data of March 2020, 41% of COVID-19 cases and 58% of deaths were observed in the Lombardia region, respectively. Conversely, less than 1% of all these COVID-19 features were registered cumulatively in the Molise and Basilicata regions. Similarly, an uneven distribution of COVID-19 cases across 104 Italian provinces was evident, with Northern Italy provinces counting the highest numbers and Southern Italy provinces the lowest numbers [22]. Several differential factors might have contributed to the uneven geographical distribution of COVID-19 cases and deaths, including population size and territorial extension, peculiar demographic characteristics, local strategies aimed at diagnosing the viral infection, spatio-temporal start and spread of COVID-19, level of adherence to strict control measures, and additional ones. In this study, we found that both population size and territorial extension (at the provincial level) were associated with COVID-19 cases in a positive (β = 0.52, p < 0.001) and negative (β = –0.32, p = 0.003) fashion, respectively. Thus, in order to minimise their confounding effect on the association between PM exposure and COVID-19 cases and deaths, the COVID-19 incidence proportion and death rate were calculated (accounting for population size), and territorial extension was always included in all multivariate models as a confounder. Our finding of a positive crude association of provincial female gender distribution with COVID-19 incidence proportion (rho = 0.20, p = 0.03; result not shown) was unexpected – Italian data from Istituto Superiore di Sanità (ISS) reported a 1.2-fold increased number of COVID-19 cases among men in the “ISS report – 2nd April 2020 – Epidemia COVID-19”. However, minimal adjustment (e.g. for only territorial extension) of the reported correlation (gender distribution vs. COVID-19 incidence proportion) abolished completely its statistical significance. In addition, gender differences in the number of COVID-19 cases disappeared in Italy with the progression of the pandemic (data available at www.epicentro.iss.it). Moreover, analysis of international gender-disaggregated data on the crude number of COVID-19 cases shows that male prevalence among COVID-19 cases is not always recurrent; accordingly, there are several countries in which women are more frequently affected by COVID-19 than men and other countries in which COVID-19 cases are equally distributed among men and women (COVID-19 sex-disaggregated data tracker. Global Health 5050. https://globalhealth5050.org/covid19/. Last data accessed on 9th May 2020). Hence, the influence of gender on COVID-19 incidence proportion remains elusive. An additional finding of the present study is that the association between the proportion of people aged ≥ 65 years and COVID-19 incidence proportion did not reach statistical significance at the provincial levels (rho = 0.17, p = 0.08); in this case, however, ISS reported that 38% of COVID-19 cases affected people aged ≥ 70 years, 17% those aged 60–70 years, and the remaining 45% those aged < 60 years, thus suggesting that a large number of people less than 65 years old may be affected by COVID-19. Finally, we failed to find associations between the above reported measures (i.e. population size, territorial extension, older age (≥ 65 years), and gender distributions) and COVID-19 death rate; this result, however, should be interpreted in light of the fact that only data for regions (total number #20) but not for provinces (total number up to #104) were available. Hence, more comprehensive and simultaneous evaluations of the independent predictivity of multiple measures (i.e. demographic and non-demographic) are necessary and awaited in order to explain the uneven geographical distribution of COVID-19 cases and deaths.
A stimulating debate has recently arisen about the hypothetical link between PM pollution and COVID-19 [21–23]. The discussion is supported by some lines of evidence and theories. First, exposure to PM pollution has been found to be particularly represented in some areas of northern Italy [24, 25], where an excess of COVID-19 cases and deaths has been reported [26]. Second, PM exposure has been causally linked to several organ dysfunctions, mostly involving the respiratory system [6–8, 27–29], the latter being responsible for the severe course of COVID-19 [2, 30]. Third, pathophysiological and epidemiological links between PM exposure and viral infections have been observed [10–20]. Fourth, recent findings of an association between indices of airborne pollution and COVID-19 incidence and fatality have been published [15, 16].
Based on these backgrounds, we wondered whether the extremely heterogeneous distribution of COVID-19 cases and deaths in Italy regions and provinces might be related to differential PM2.5 and PM10 exposure. We showed a significant relationship between all PM exposure indices and both COVID-19 incidence proportion and cause-specific death rate. Importantly, these associations were robust after adjustment for several confounders. In addition, the results were consistent across different indices of PM exposure. Based on these results we may assume that exposure to PM2.5 and PM10 might be considered as possible determinants of COVID-19 onset and severity.
According to published literature, several PM-related mechanistic actions leading to increased vulnerability to SARS-CoV-2 may be proposed, including: 1) dysregulation of early-phase immune response leading to reduced immune defences [10, 17]; 2) inflammation and injury of airways [18, 31]; 3) exacerbation of pre-existing disorders (e.g. chronic obstructive pulmonary disease, asthma, cardiovascular diseases, etc.) [19, 32]; 4) activation of the systemic inflammation cascade [33, 34]; and 5) formation of PM-viral complexes facilitating viral spread [20]. Each of these putative mechanisms linking PM exposure to COVID-19 are highly intriguing although still undemonstrated.
Limitations of this study should be acknowledged. The intrinsic weaknesses of all ecological studies are applicable to this study as well. In this regard, the risk of ecological fallacy leading to biased interpretation of the results may occur due to the possible generalisation of the average measures of exposure to the individual level. Therefore, caution should be exercised when interpreting the results of the present study. Also, potential bias deriving from geographical systematic differences in recording COVID-19-related frequencies or PM exposures may have occurred. However, homogeneous data collection from the Italian Ministry of Health has been performed in all regions and provinces during the COVID-19 outbreak. In addition, multiple indices of PM exposure have been collected. Thus, the latter limitation may have been attenuated. Moreover, the lack of data on potentially important confounders of the association between PM exposure and COVID-19 frequencies should be considered. Thus, a more comprehensive adjustment for additional demographic and disease-related factors is awaited. A further limitation of this study is that PM2.5 and PM10 exposure data are relative to 2017, possibly expressing past exposure to PM pollution. More recent data on these measures were not publicly available. Hence, whether significant changes over time in regional and provincial PM pollution exposure occurring since 2017 until today had an impact on the present results is uncertain.
In conclusion, SARS-CoV-2 infection in Italy affected and caused the death of an extremely high number of subjects, with a heterogeneous distribution across different regions and provinces. We provide data suggesting that the greater diffusion and aggressiveness of COVID-19 might be related, at least in part, to the concomitant past and cumulative exposure to PM pollution. Long-term strategies aimed at protecting people from outdoor PM pollution are eagerly awaited, along with the development of specific and effective therapies and vaccines [3, 35].