Abstract
Background and Aims
COVID-19 vaccine hesitancy varies across the USA. Data on COVID-19 vaccine hesitancy in patients with inflammatory bowel disease (IBD) are lacking. We assessed COVID-19 vaccine hesitancy and its associated variables in patients with IBD.
Methods
We evaluated voluntary patient survey responses during routine clinical visits to our IBD center. Data collected included demographic and clinical characteristics. Descriptive statistics, univariate and multivariate analyses were performed to evaluate significant associations with COVID-19 vaccine hesitancy.
Results
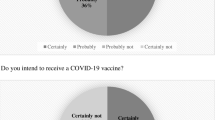
A total of 239 individuals completed the survey. Over a third of respondents (35.6%) expressed hesitancy toward receiving the COVID-19 vaccine due to vaccine safety concerns (49.4%) and efficacy (23.5%), while others reported non-specific concerns (34.1%). On univariate analysis, Crohn’s disease (OR 2.33 CI 1.28–4.25 p = 0.0056), use of biologic medications (OR 1.93 CI 1.16–3.23, p = 0.012), previous self-reported vaccine refusal (OR 8.13 CI 2.90–22.82 p = 0.0001), earlier date of survey administration (OR 2.01 CI 1.17–3.44 p = 0.011), and self-reported COVID infection (OR 2.55 CI 1.16–5.61 p = 0.0056) were more likely to be associated with COVID-19 vaccine hesitancy. On multivariate analysis, patient age, previous vaccine refusal and date of survey administration were more likely to be associated with COVID-19 vaccine hesitancy.
Conclusions
Over one-third of patients with IBD expressed COVID-19 vaccine hesitancy. Vaccine safety and efficacy were the most common reasons. Younger age, previous vaccine refusal and earlier date of survey were more likely to be associated with hesitancy. Our findings suggest that there is room for targeted education to improve COVID-19 vaccine uptake in patients with IBD.
Similar content being viewed by others
Introduction
Approximately, 51–62% of the general population in the USA expresses some hesitancy in receiving the COVID-19 vaccine. The true estimate varies based on location, culture and timing of sampling through the COVID pandemic [1]. Previously reported factors associated with hesitancy include lack of a college degree, lower income, lack of insurance, rural residence, larger households and black race. Other demographic factors such as age and gender have mixed results based on location of survey and specific study. However, the overall trend shows that female and younger patients are generally more hesitant [2].
Multiple stakeholders, clinicians, professional societies and thought leaders involved in the care of patients with IBD recommend COVID-19 vaccination for nearly all patients. A recent survey of gastroenterologists also indicates an almost universal agreement on recommending COVID-19 vaccination [3, 4]. However, patients with IBD have understandable concerns about the risks of COVID-19 vaccination and the potential impact on their disease course. To date, few of studies have quantified hesitancy in patients with IBD. In the USA, a single study has found hesitancy rates of 19.1% in an office-based cohort and 40% in a social media cohort. Two other studies outside of the USA have found hesitancy rates in office-based cohorts of 17.7% in Italy and 45.2% in France [5,6,7]. While multiple of these studies have found associations between lower educational achievement and vaccine hesitancy, results differ on the relationship between gender, age and IBD therapy type and COVID-19 vaccine hesitancy.
Subsequently, there have been calls for further research to understand the barriers to vaccination in patients with IBD [4]. We sought to evaluate the extent of and factors associated with COVID-19 vaccine hesitancy in patients with IBD receiving care at our tertiary hospital multidisciplinary IBD center.
Methods
Study Design and Participants
We administered a short survey to all patients with IBD who presented to our multidisciplinary IBD center for care from 01/14/2021 to 05/10/2021. The survey contained questions on previous vaccine hesitancy, willingness to take the COVID-19 vaccine, and reservations about the vaccine, if any (Supplemental Fig. 1). Basic demographic data on age, gender, level of education, religion as well as disease-specific data such as type of IBD (Crohn’s Disease vs. Ulcerative Colitis) and IBD treatment (biologic, immunosuppressive, steroid) were extracted from the PSH IBD consented Registry, as well as from the electronic medical record review.
Hesitancy was defined to include responses of being undecided, somewhat likely to refuse or refusing the vaccine. Patients considered non-hesitant responded that they were somewhat likely to or will get the vaccine, or already had received the vaccine.
Inclusion Criteria
-
Adult patients (≥ 18) with an established diagnosis of IBD receiving care at our health center.
-
Attended an in-person appointment between 01/2021 and 05/2021.
Patients were excluded if they declined to participate or were under the age of 18.
All participating patients were also part of the consented Inflammatory Bowel Disease Registry.
Statistical Analyses
Results are descriptive and reported as percentages. For univariate analysis, continuous variables such as age and date of survey administration were dichotomized into categorical variables based on median value. Univariate analysis was conducted using MedCalc’s desktop suite version 20.008. RStudio was used for multivariate analysis in which an ordinal logistic regression of all variables that were statistically significant on univariate analysis was conducted.
Results
Baseline Patient Characteristics
There were 443 patients who were approached for participation in the study, of which 239 patients fully completed the survey and were included in the analysis (54.0%). Approximately, one-third of respondents (35.6%) expressed hesitancy toward receiving the COVID-19 vaccine. The median age of participants was 42 (IQR 29.5–54), 49.8% were male and 86.6% were white. Of the participants included in the analysis, 64.1% had Crohn’s disease and 34.7% had ulcerative colitis. (Table 1).
Patient Hesitancy Rates
Of those that were hesitant, 49.4% were concerned about the vaccine’s safety, 23.5% about its’ efficacy and 34.1% reported they did not have concerns. Over half (55.6%) of all respondents reported that they will receive, were somewhat likely to receive or had already received the vaccine.
Of those that were not hesitant, 16.2% reported concerns over safety, 11.7% reported concerns over efficacy, while 72.7% had no concerns. Furthermore, 15.3% of hesitant patients and 5.8% of non-hesitant patients had other concerns (Supplemental Table 1).
Factors Associated with Vaccine Hesitancy
On univariate analysis, CD (OR 2.33 CI 1.28–4.25 p = 0.0056), use of biologic medications (OR 2.02 CI 1.18 p = 0.0106), previous vaccine refusal (OR 8.13 CI 2.90–22.82 p = 0.0001), earlier date of survey administration (OR 2.01 CI 1.17–3.44 p = 0.011), low population density zip code (OR 1.72 CI 1.01–2.94 p = 0.046) and self-reported COVID infection (OR 2.55 CI 1.16–5.61 p = 0.0056) were more likely to be associated with COVID-19 vaccine hesitancy (Table 2). To further explore the relationship between time of survey administration and vaccine hesitancy, we divided our period of survey administration into quartiles (Table 3).
The use of 5-ASA (OR 0.34 CI 0.18–0.62 p = 0.0005), older age (OR 0.33 CI 0.19–0.58 p = 0.0001) and Catholic religion (OR 0.36 CI 0.13–0.99 p = 0.047) was less likely to be associated with COVID-19 vaccine hesitancy. Steroid use within last 30 days, steroid use within past year, disease duration, previous history of surgery for IBD, race, ethnicity and marriage status were not significantly associated with risk of vaccine hesitancy.
On multivariate analysis through ordinal logistic regression of the statistically significant variables, the effect of previous vaccine refusal, earlier date of survey administration and age remained statistically significant (Table 4).
Discussion
In general, vaccines are underutilized in patients treated with immunosuppressive regimens [8]. Evidence from early studies during the pandemic indicated that patients with rheumatologic diseases are more hesitant about taking the COVID-19 vaccine compared to the general population (45.1 vs. 17.7%, OR 4.16 95% CI 2.94–5.88) independent of the type of treatment they received [9]. However, patients with IBD were not included in the aforementioned analysis. Emerging evidence shows that patients with IBD may be less likely to get the vaccine in Germany, and may be more likely to delay receiving it until they know more about its side effects [10].
In pre-pandemic studies of patients with IBD, significant predictors of recommended vaccination completion rates were annual vaccination review by family physician or gastroenterologist, current or prior treatment with biologicals and current steroid use [11]. Another study found higher education level to be independently associated with adherence to pneumococcal vaccination and other guidelines [12]. The study by Narula et al. [13] found that the frequency of H1N1 vaccination was high among patients with IBD who visited their primary care practitioner (PCP) at least once annually. Specific demographic factors associated with poor uptake of common vaccines reported by a survey-based study include lower education level, younger age, and lack of chronic immunosuppression use [14]. While there is evidence that family medicine practitioners may not be comfortable managing vaccines for immunosuppressed patients, it is not clear if this extends to the COVID-19 vaccines [15].
It is difficult to directly compare pre-pandemic vaccine hesitancy in patients with IBD to our results. The referenced studies measured completion of vaccines, whereas our analysis is limited to intent and hesitancy. To date, there is one study evaluating COVID-19 vaccine intent in patients with IBD in the USA. [5]. That study included two cohorts: one of patients at Brigham and Women’s Hospital (BWH) and another of social media participants. Hesitancy was noted in 19.1% of the cohort at BWH and 40% in the social media cohort, while our study noted hesitancy in 35.6%. Notably, their sampling took place over a month in January of 2021, whereas our sampling occurred over 4 months starting in January of 2021. Their definition of hesitancy was more expansive and included patients that “will likely receive the vaccine, but at a later time.” Despite the differences in timing and definition of hesitancy, their finding that older patients were more intent on taking the vaccine mirrors our finding of less hesitancy in older patients (OR 0.33).
There are notable differences between our findings and those of Dalal et al. [5], especially when evaluating the results of the social media cohort. Whereas they found that vaccine intent was higher in patients in the social media cohort on biological therapies (OR 1.5), we found that patients on biological therapies were more hesitant about the COVID-19 vaccines (OR 2.02), and those on ASA were less hesitant (OR 0.34) on univariate analysis. This may reflect increased concern among patients with more severe disease throughout the vaccine rollout or differences in the populations surveyed. Notably, these findings were not statistically significant on multivariate analysis.
We also found patients with self-reported COVID-19 infections were more hesitant than those not self-reporting infections (OR 2.52) on univariate analysis, where participants in the social media cohort with self-reported infections were more intent on taking the vaccine (OR 2.0). We postulate that may be a result of differences in the population participating over social media compared to those participating in-person. Alternatively, it may reflect the attitudes of central Pennsylvanians that attend our IBD clinic differing from nationwide social media participants.
While our analysis was limited by a small sample of non-white participants (13.4%), we did not find a significant difference in vaccine hesitancy between racial groups but did find whites to be more likely to have vaccine intent (OR 2.1). Our study did not assess level of education, which Dalal et al. [5] noted to be associated with vaccine intent.
Our analysis of religious denominations was based on patients’ responses at the time of their first encounter with our health system. The only religion / denomination that showed significant difference in hesitancy was Catholic (OR 0.36, p = 0.047). The average age of the Catholic cohort was 49.3 (± 15.1) years, which may have contributed to this group’s acceptance of the vaccines. In addition, the US Conference of Catholic Bishops recommended vaccination in December of 2020 despite their reservations on the use of fetal cell lines [16]. Finally, Catholic was the largest denomination identified, following No Religious Preference. It is possible more denominations and religions would have shown significant vaccine hesitancy or intent with a larger sample size. More work is needed to confirm these observations.
Participants responding before the median date of survey administration, March 5, 2021, were more likely to be hesitant about the vaccine, than those responding later (OR 2.01), which retained significance on multivariate analysis. This difference occurred without any educational interventions targeted at reducing hesitancy during the clinic visit. We attribute part of this difference to external factors that may have influenced individual decision making. The beginning of the survey (January 2021) coincided with vaccine rollout, whereas by the end (May 2021), over one-third of the USA had one dose of vaccine.
Outside of the USA, Caron et al. [6] found that 45.2% of 104 surveyed local patients with IBD in France from February to January were certainly or probably not going to take the COVID-19 vaccine. In Italy, an online national survey conducted on 1252 patients with IBD in February 2021 by Costantino et al. [7] found a much lower vaccine hesitancy rate of 17.7%. That study found positive attitudes toward vaccination, male gender, higher education level, perceived higher risk of infection due to IBD and alcohol intake to be associated with decreased hesitancy. Positive attitudes about complementary medicine was associated with increased hesitancy. Similar to our findings, attitudes toward vaccination in general was the most predictive of hesitancy, with an adjusted OR of 17.6. In addition, their results did not find a relationship between age and vaccination hesitancy.
Commonly reported reasons for pre-pandemic vaccine hesitancy among patients with IBD include uncertainty about indications, fears of immediate side effects or delayed complications, as well as concerns regarding vaccine safety and fear of precipitating an IBD flare [11, 13]. Similar to other studies, our results indicate that patients with hesitancy were most frequently concerned about long-term safety [5,6,7].
While the evidence supporting increased risk of severe COVID-19 is limited, it has been shown that persons with IBD who received steroid therapy three months earlier are at higher risk of severe COVID-19 [18]. Furthermore, when patients with IBD on immunosuppressive therapy are vaccinated against diseases like HBV, their serological response may not be as vigorous [8, 19]. There is preliminary evidence showing that patients on infliximab and vedolizumab develop adequate antibody titers less frequently after COVID-19 infection and have sub-optimal responses to the COVID-19 vaccines [20, 21]. Thus, it is increasingly important that patients with IBD are vaccinated and studied to ensure that recommendations about boosters are evidence-based.
Guidelines about third doses and boosters to COVID-19 vaccines are rapidly changing. In Britain in September, the Joint Committee on Vaccination and Immunization recommended third primary doses for patients on immunosuppressive or immunomodulating therapy such as TNF-a inhibitors and high-dose corticosteroids at the time of initial vaccination [22]. Later, in October, the Advisory Committee on Immunization Practices issued a similar recommendation in the USA [23]. Further work is needed to characterize hesitancy related to third and booster shots as well as their efficacy in patients with IBD both on and off of immunosuppressive therapies [24].
There may be opportunities to intervene and increase vaccine uptake in patients with IBD. Pre-pandemic studies have shown that administering a 1-page questionnaire about vaccine coverage to patients in clinic followed by offering missing vaccines can dramatically improve vaccine uptake among patients with IBD [25]. Additionally, educational interventions provided by nurses using brochures and vaccination cards can increase vaccine uptake in the outpatient setting [12]. Our results suggest that interventions should target patients that have previously refused vaccines and younger patients. Future studies are needed to investigate whether patients on biological therapies, CD patients and those self-reporting infections with COVID-19 are at increased risk of vaccine hesitancy, given our findings on univariate analysis.
Our study has some limitations. The survey was limited to four questions, and did not include questions about specific vaccine formulations or producers. While previous refusal of vaccinations was asked, it was not determined which vaccines or how many vaccines persons had refused. Furthermore, vaccine refusal and previous COVID-19 infection were self-reported, and could not be verified through chart review. Our survey administration time frame spanned eras of differing public perception of the vaccine, which may have inadvertently affected responses. Participation in our survey was limited to in-person patient encounters at a single academic tertiary care center and may be skewed toward more complicated patients and predominantly white patients. The survey excluded those patients that had telemedicine appointments during the dates the survey was administered. When analyzed, the telemedicine patient group (n = 121) had similar age, (average age 42 years) and diagnoses (69% CD) though there was a smaller proportion of male patients (35% vs 49% in-person). Our survey completion rate of 54% is comparable to average response rates of other surveys [26]. Patients that declined to fill out the survey showed similar average age (42 yrs), IBD diagnosis (62% CD) and gender (46% male) (Table 5). The reasons for declining the survey were not recorded, but ranged from time constraints, paperwork fatigue to conscious declination.
A little over a third of patients with IBD (35.6%) were hesitant to take the COVID-19 vaccine in this single-center prospective cohort study. Previous vaccine hesitancy, younger age and early date of survey administration were associated with higher vaccine hesitancy in patients with IBD on multivariate analysis. Vaccine hesitancy decreased over the course of January 2021 to May 2021. We postulate that the temporal decline in vaccine hesitancy may be related to patients reviewing more available information about the vaccines as well as targeted discussions during clinic visits. Targeted interventions may help increase vaccination uptake in the IBD population.
References
Lin C, Tu P, Beitsch LM. Confidence and receptivity for covid-19 vaccines: a rapid systematic review. Vaccines. 2021;9:16.
Sallam M. COVID-19 vaccine hesitancy worldwide: a concise systematic review of vaccine acceptance rates. Vaccines. 2021;9:160.
Siegel CA, Melmed GY, McGovern DPB et al. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: recommendations from an international consensus meeting. Gut. 2021;70:635.
Alexander JL, Moran GW, Gaya DR et al. SARS-CoV-2 vaccination for patients with inflammatory bowel disease: a British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement. Lancet Gastroenterol Hepatol. 2021;6:218–224.
Dalal RS, McClure E, Marcus J, Winter RW, Hamilton MJ et al. COVID-19 vaccination intent and perceptions among patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2021;19:1730-1732.e1732.
Caron B, Neuville E, Peyrin-Biroulet L. Inflammatory bowel disease and COVID-19 vaccination: a patients’ survey. Dig Dis Sci. 2021. https://doi.org/10.1007/s10620-021-07040-z.
Costantino A, Noviello D, Conforti FS, et al. COVID-19 Vaccination willingness and hesitancy in patients with inflammatory bowel diseases: analysis of determinants in a national survey of the Italian IBD patients' association. Inflamm Bowel Dis. 2021;izab172.
Melmed GY, Ippoliti AF, Papadakis KA et al. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol. 2006;101:1834–1840.
Priori R, Pellegrino G, Colafrancesco S et al. SARS-CoV-2 vaccine hesitancy among patients with rheumatic and musculoskeletal diseases: a message for rheumatologists. Ann Rheum Dis. 2021;80:953–954.
Walldorf J, von Arnim U, Schmelz R et al. SARS-CoV-2 vaccination in patients with inflammatory bowel disease-fear and desire. Inflamm Bowel Dis. 2021;27:1858–1861.
Malhi G, Rumman A, Thanabalan R et al. Vaccination in inflammatory bowel disease patients: attitudes, knowledge, and uptake. J Crohns Colitis. 2015;9:439–444.
Coenen S, Weyts E, Jorissen C et al. Effects of education and information on vaccination behavior in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:318–324.
Narula N, Dhillon AS, Chauhan U, Marshall JK. An audit of influenza vaccination status in adults with inflammatory bowel disease. Can J Gastroenterol. 2012;26:593–596.
Wasan SK, Calderwood AH, Long MD, Kappelman MD, Sandler RS et al. Immunization rates and vaccine beliefs among patients with inflammatory bowel disease: an opportunity for improvement. Inflamm Bowel Dis. 2014;20:246–250.
Selby L, Hoellein A, Wilson JF. Are primary care providers uncomfortable providing routine preventive care for inflammatory bowel disease patients? Dig Dis Sci. 2011;56:819–824.
Moral Considerations Regarding the New COVID-19 Vaccines, 2020. Available at: https://www.usccb.org/moral-considerations-covid-vaccines. Accessed 11/23/2021.
Olagoke AA, Olagoke OO, Hughes AM. Intention to vaccinate against the novel 2019 coronavirus disease: the role of health locus of control and religiosity. J Relig Health. 2021;60:65–80.
Singh S, Khan A, Chowdhry M, Bilal M, Kochhar GS et al. Risk of severe coronavirus disease 2019 in patients with inflammatory bowel disease in the United States: a multicenter research network study. Gastroenterology. 2020;159:1575-1578.e1574.
Reich J, Wasan S, Farraye FA. Vaccinating patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2016;12:540–546.
Kennedy NA, Goodhand JR, Bewshea C et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70:865–875.
Edelman-Klapper H, Zittan E, Bar-Gil Shitrit A, et al. Lower Serologic Response to COVID-19 mRNA Vaccine in Patients With Inflammatory Bowel Diseases Treated with Anti-TNFalpha. Gastroenterology. 2021;S0016–5085(0021)03701-X.
Department of Health & Social Care. Joint Committee on Vaccination and Immunisation (JCVI) advice on third primary dose vaccination, 2021. Available at: https://www.gov.uk/government/publications/third-primary-covid-19-vaccine-dose-for-people-who-are-immunosuppressed-jcvi-advice/joint-committee-on-vaccination-and-immunisation-jcvi-advice-on-third-primary-dose-vaccination. Accessed 11/23/2021.
Mbaeyi S, Oliver S, Collins J et al. The advisory committee on immunization practices’ interim recommendations for additional primary and booster doses of COVID-19 vaccines — United States. MMWR Morb Mortal Wkly Rep. 2021;70:1545–1552.
Doherty J, Fennessy S, Stack R et al. Review Article: vaccination for patients with inflammatory bowel disease during the COVID-19 pandemic. Aliment Pharmacol Ther. 2021;54:1110–1123.
Parker S, Chambers White L, Spangler C et al. A quality improvement project significantly increased the vaccination rate for immunosuppressed patients with IBD. Inflamm Bowel Dis. 2013;19:1809–1814.
Nakash RA, Hutton JL, Jørstad-Stein EC, Gates S, Lamb SE. Maximising response to postal questionnaires–a systematic review of randomised trials in health research. BMC Med Res Methodol. 2006;6:5.
Funding
None to report.
Author information
Authors and Affiliations
Contributions
KC study conception and design, development of survey, oversight and editing of manuscript. MP manuscript writing, statistical analysis. AS development of survey, manuscript writing. AT manuscript review and editing. SD manuscript review and editing. NB manuscript review and editing. EW manuscript review and editing. MC manuscript review and editing.
Corresponding author
Ethics declarations
Conflicts of interest
KC has reviewed research grants for Pfizer, Participated in Speakers Bureau for Janssen, ABBVie, Takeda and Pfizer. EW Participated in Speakers Bureau for Pfizer and ABBVie. AT Participated in Speakers Bureau for Pfizer. NB Participated in Speakers Bureau for Pfizer and AbbVie.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki and approved by the Pennsylvania State University College of Medicine Institutional Review Board and carried out under protocol STUDY00013788.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Clarke, K., Pelton, M., Stuart, A. et al. COVID-19 Vaccine Hesitancy in Patients with Inflammatory Bowel Disease. Dig Dis Sci 67, 4671–4677 (2022). https://doi.org/10.1007/s10620-021-07377-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-021-07377-5