Abstract
Purpose
To evaluate the possible effects of novel coronavirus disease 2019 (2019-NCOV) on male sex hormones and reproductive ability, and analyze its incidence and risk factors.
Methods
We retrieved from PubMed, Embase, The Cochrane Library, Web of Science, Clinical Trails, CNKI, CBM, Wan Fang Database and VIP to collect research on the effects of COVID-19 on the male sex hormone. Our literature search was conducted until April 2022, and two investigators independently screened articles based on inclusion and exclusion criteria. In strict accordance with the inclusion and exclusion criteria, two researchers independently screened the literature and comprehensively analyzed 8 cohort studies on the impact of COVID-19 on male sex hormone. And We used RevMan5.4.1 and Stata15.0 for statistical analysis. Finally, there were eight cohort studies on the effects of COVID-19 on male sex hormones.
Results
T(RR = − 3.94; 95% CI − 6.22, − 1.66; P = 0.0007), testosterone in the COVID-19 group decreased by 3.94 nmol/L compared with the control group, and the difference was statistically significant. LH (RR = 0.85; 95% CI − 0.26, 1.96; P = 0.13), the LH in COVID-19 group was 0.85 mlU/ml higher than that in control group, but the difference was not statistically significant. FSH (RR = 0.25; 95% CI − 0.72, 1.23; P = 0.61), the FSH of COVID-19 group was 0.25 mlU/ml higher than that of the control group, but the difference was not statistically significant. PRL (RR = 2.42; 95% CI 0.52, 4.31; P = 0.01), the PRL in the COVID-19 group was 2.42 ng/ml higher than that in the control group, and the difference was statistically significant. E2(RR = 11.88; 95% CI 9.90, 13.86; P < 0.00001), The level of E2 in the COVID-19 group was 11.88 pg/ml higher than that in the control group, and the difference was statistically significant. T:LH (RR = − 0.39; 95% CI − 076, − 0.02; P = 0.04), the ratio of T:LH in COVID-19 group was lower than that in control group, and the difference was statistically significant. FSH:LH (RR = − 0.38; 95% CI − 0.86, 0.11; P = 0.13), the ratio of FSH:LH decreased in COVID-19 group compared with control group, but the difference was not statistically significant.
Conclusions
COVID-19 can affect the level of sex hormones, especially T, which may further affect male fertility. Due to the limitations of this study, this conclusion needs to be further verified by large-sample, high-quality prospective cohort studies on the long-term effects of COVID-19 on male sex hormones and fertility.
Similar content being viewed by others
1 Introduction
Since December 2019, Corona Virus Disease 2019 (COVID-19) has erupted globally. As of April 24, 2022 (Beijing time), the number of confirmed COVID-19 cases worldwide has exceeded 440 million, and the cumulative number of deaths has exceeded 6 million. The domestic and foreign research shows that the effects of COVID-19 on male sex hormones are still controversial. It is believed that SARS-CoV-2 can cause substantial damage to male testicular tissue and induce changes in male sex hormone levels [1]. In the observational study by Apaydin et al. [2], most patients had low testosterone levels, and in the later 6-month follow-up, nearly half of patients still had testosterone levels below normal. Some patients’ testicles are infected with the SARS-CoV-2 virus, which is manifested by hypogonadism [3].
Testis produce sex hormones, and the normal physiological structure and function of testis are also the prerequisite for male reproduction. With the opening of the three-child policy, whether it will have an impact on male fertility is also an urgent concern of people. Given the potential impact and unknown outcomes of COVID-19 on the male reproductive system, it is necessary to determine reproductive outcomes in men with clinical symptoms or confirmed COVID-19. This study adoptedd the method of evidence-based medicine to collect published literature, objectively evaluate the impact of COVID-19 on sex hormones, and formulate corresponding defense measures to provide high-quality basis for primary prevention and health care services.
2 Methods
2.1 Scheme and Registration
Registered with PROSPERO as CRD42020181812.
2.2 Search Strategy
PubMed, Embase, The Cochrane Library, Web of Science, Clinicaltrails, CNKI, CBM, Wanfang Database and VIP database were searched by computer. PubMed, Embase, The Cochrane Library, Web of Science, Clinicaltrails, CNKI, CBM, Wanfang Database and VIP database were searched by computer. All database literature is from inception to April 2022. Using the combination of free words and subject words, the search language is not limited. Search words include: SARS-CoV-2, COVID-19, Coronavirus Disease 2019 Virus, 2019 Novel Coronaviruses, Gonadal Hormones, male sex hormones, testosterone, luteinizing hormone, Follicle Stimulating Hormone, male reproductive system, etc.
2.3 Inclusion and Exclusion Criteria
Inclusion criteria: (1) Published literature or ongoing clinical trials data; (2) Cohort studies or case–control studies with COVID-19 and control groups, whether or not double-blind; (3) Patients in the experimental group were at the stage of COVID-19 infection or recovery. The control group was non-COVID-19 normal healthy people; (4) The variable detection methods in literature were consistent.
Exclusion criteria: (1) No control group or poor balance between groups, no comparability; (2) The research contents are inconsistent or do not meet the inclusion criteria; (3) Cross-sectional studies, reviews, experience summaries, theoretical discussions, experimental animal studies, case reports, and repeated published studies; (4) The experimental group or the control group had infections other than COVID-19, drugs affecting the HPA axis, hypothalamic-pituitary diseases treated with hormone therapy, chemotherapy with immunosuppressive drugs, etc. (5) The full text cannot be obtained or the outcome indicators are inconsistent; (6) No valid data were extracted or there were obvious errors in the data.
2.4 Literature Quality Assessment
The Newcastle–Ottawa Scale (NOS) was used to evaluate the quality of the literature of prospective cohort studies, which mainly consisted of the following three aspects: (1) The selection of the study population (4 points): ①whether the exposure group is representative: 1 point from a random sample of the general population, no points from special groups, such as nurses, volunteers, etc.; ② Selection of non-exposed group: 1 point for being from the same population as the exposed group, otherwise no points; ③ Confirmation of exposure: 1 point for patients diagnosed with COVID-19 by definite nasopharyngeal swabs or oral specimens receive; otherwise no points; ④ 1 point for absence of urinary tract disease and COVID-19 symptoms prior to follow-up, otherwise no points. (2) Comparability between groups (2 points): whether the relevant confounding factors were adjusted or not: 2 points for adjusting age and important confounding factors related to the disease, 1 point for adjusting age or important confounding factors related to the disease, and no points for not adjusting either. (3) Result evaluation (3 points): ① Evaluation of outcome: 1 point for clear recording of sex hormone data, otherwise no score; ② Follow-up years: 1 point for more than 60 days of follow-up, otherwise no points; ③ Cohort population lost visit rate: less than 25% scored 1 point, otherwise no score. The results of the literature scoring are shown in Table 2. NOS scores out of 9, with 0 to 3, 4 to 6, and 7 to 9 being low, medium, and high quality studies in that order.
2.5 Literature Screening and Data Extraction
The data will be collected and screened by two assessors independently. In case of divergence, it will be resolved through discussion. If necessary, a third researcher will make the judgment. The extracted content includes the publication year, the first author, the research center, the total number of patients in the control group and the experimental group, the average age, clinical symptoms, BMI (Body Mass Index), and outcome indicators. After data extraction, the third researcher will check the extracted results. If there is any discrepancy in the data, it will be processed through group discussion or consultation with professional statisticians. In addition, for the analysis of sex hormone levels, we will collect the following data: testosterone (T, nmol/L), luteinizing hormone (LH, mlU/ml), follicle-stimulating hormone (FSH, mlU/ml), prolactin (PRL, ng/ml), estradiol (E2, pg/ml).
2.6 Statistical Methods
We will use RevMan 5.4.1 tool to draw forest plot, and use Stata17.0 software to generate funnel plot and Egger’s test. When evaluating heterogeneity among studies, we will adopt Q value and I2 value. When I2 ≤ 50% or P ≥ 0.1, it indicates no significant heterogeneity. Subsequently, we will calculate the P value, RR value and its 95% confidence interval of the combined statistics. To further evaluate publication bias, we will use funnel plot and Egger’s test. If the P ≥ 0.05, it indicates no significant publication bias. For data that cannot be processed by meta-analysis, we will use descriptive statistics for analysis.
3 Results
3.1 Screening Process and Eligible Studies
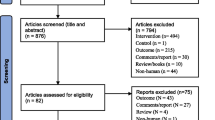
A total of 1716 studies were included. After reading the title, abstract and full text, and excluding the articles that did not meet the inclusion criteria, 8 articles were finally included, all of which were prospective cohort studies in English. The literature screening process and results are shown in Fig. 1.
3.2 Study Characteristics
A total of eight studies were included in this meta-analysis, including three from China, one from the Russia, two from Turkey, and the other two are not recorded. A summary of the included studies is presented in Table 1. (The experimental group is in the front, and the healthy group is in the back).
3.3 Quality Evaluation of the Included Studies
The included articles were all prospective cohort studies, and their risk of bias was evaluated according to the NOS scale. Eight of the included literatures had the study score of ≥ 6 stars, and the quality of the included studies was high. The specific scores are shown in Table 2.
4 Results of Meta-Analysis on Sex Hormones
4.1 Testosterone
There are 8 articles about the effect of COVID-19 on testosterone [4,5,6,7,8,9,10,11], and testosterone was 3.94 nmol/L lower in the COVID-19 group than in the healthy control group (overall MD − 3.94; 95% CI − 6.22, − 1.66; Z = 3.39, P = 0.0007) (Fig. 2). However, MDs between-study were highly varied (I2 = 96%, P < 0.00001).
4.2 Luteinizing Hormone
According to the meta-analysis from 8 [4,5,6,7,8,9,10,11], Luteinizing hormone of those subjects COVID-19 was 0.85 more than those healthy control group (overall MD: 0.85; 95% CI − 0.26, 1.96; Z = 1.50, P = 0.13 > 0.05) (Fig. 2), but the difference was not statistically significant.
4.3 Follicle Stimulating Hormone
Meta-analysis from 8 studies [4,5,6,7,8,9,10,11] revealed that Follicle Stimulating Hormone COVID-19 was 0.25 mlU/ml more than those healthy control group (overall MD 0.25; 95% CI − 0.72, 1.23; Z = 0.51, P = 0.61 > 0.05) (Fig. 2). However, its MD was highly varied.
4.4 Prolactin
MD from 5 studies [5,6,7, 10, 11] about Prolactin were highly varied (I2 = 85%, P = 0.0001). There was statistically significant pooled MD (95% CI) of 2.42 (0.52, 4.31) (Z = 2.50, P = 0.01) suggesting that the prolactin of those COVID-19 group was 2.42 ng/ml higher than these healthy control group (Fig. 2).
4.5 Estradiol
According to two studies [4, 11], estradiol level among those COVID-19 group was more than that among healthy control groups with overall MD (95% CI) of 11.88 (9.90, 13.86) (Z = 11.75, P < 0.00001) with presence of low heterogeneity (I2 = 0%, P = 0.4).
4.6 T: LH
T: LH among those COVID-19 group is less than that in healthy control group overall MD − 0.39, 95% CI (− 076, − 0.02), Z = 2.07, P = 0.04 (Fig. 2). MD between these 2 studies [8, 10] were highly varied (I2 = 85%, P = 0.1).
4.7 FSH: LH
MD about FSH: LH from 2 studies [8, 10] were highly varied (I2 = 87%, P = 0.006) (Fig. 2). The results showed that the COVID-19 group was 0.38 lower than the healthy control group, but the difference was not statistically significant. (Overall MD: − 0.38; 95% CI – 0.86, 0.11; Z = 1.52, P = 0.13 > 0.05).
5 Sensitivity Analysis and Subgroup Analysis
As analyzed above, there was great heterogeneity among studies. When the literature was removed one by one for sensitivity analysis, the pooled results of the remaining studies did not change significantly, indicating that the research results were relatively robust. We considered that the heterogeneity might be caused by differences in age and clinical characteristics. When we conducted subgroup analysis based on age differences, we found that the heterogeneity among subgroups was still large, and the differences in the combined results were different, indicating that age may not be the source of the differences (Fig. 3). Due to the diversity of clinical features, it is impossible to carry out subgroup analysis, which may be one of the sources of differences.
6 Test of Publication Bias
In this systematic review, we simultaneously used funnel plot and Egger’s test to analyze publication bias of articles. The funnel plots in most studies are asymmetric and uneven (Fig. 4). The reason may be that the number of eligible studies in the meta-analysis in this study is relatively small and the research index are different, which may lead to a further reduction in the number of included studies. At the same time, some sample sizes of some articles are small, and there are regional differences and heterogeneity.
However, Egger’s test showed that there was no publication bias between the COVID-19 group and the healthy control group, and the results were as follows. T: coefficient = 3.965, SE = 5.53, P = 0.5 > 0.05 (Fig. 5a). LH: coefficient = − 3.432, SE = 3.466, P = 0.36 > 0.05 (Fig. 5b). FSH: coefficient = − 0.482, SE = 2.161, P = 0.831 > 0.05 (Fig. 5c). PRL: coefficient = − 1.449, SE = 3.376, P = 0.697 > 0.05(Fig. 5d). There are three studies such as E2, T: LH and FSH: LH included too few literatures to make Egger’s test chart.
7 Discussion
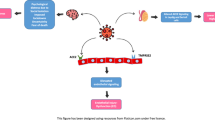
Sex hormones and the ratio of sex hormones are closely related to semen quality, sperm fertilization ability and fertility [12, 13]. And low levels of sex hormones may lead to increased sperm DNA damage, further increasing the possibility of male infertility [14]. The invasion of SARS-CoV-2 is mainly related to human angiotensin converting enzyme 2 (ACE2) and type II transmembrane serine protease (TMPRSS2). Androgens are mainly synthesized in interstitial cells, and ACE2 and TMPRSS2 are expressed in interstitial cells [15, 16]. Study has shown that testosterone can regulate the expression of ACE2 and TMPRSS2 simultaneously [17], which may promote the internalization of SARS-CoV-2 [18]. And compared to estrogen, testosterone may make men more susceptible to COVID-19 than women [18, 19].
Since basal T levels may vary considerably in populations, the ratio between hormones such as T: LH and FSH: LH is considered to be a better parameter for assessing male gonadal function [8]. The decrease of T level and T:LH ratio in this study indicates that COVID-19 will affect the level of male sex hormones, leading to hypogonadism and even infertility [2, 20]. There are different views on the mechanism of COVID-19 affecting T level. Sengupta et al. believe that SARS-CoV-2 damages leydig cells, resulting in the synthesis of T and other hormones [21]. However, Adel believed that SARS-CoV-2 directly affected the production of sex hormones and the hypothalamic-pituitary–testicular axis, ultimately leading to primary leydig cell injury [22]. The mechanism by which COVID-19 affects hormone levels remains to be further identified.
We analyzed the reasons for the high heterogeneity of this systematic review. First, BMI has an effect on male hormones [23, 24], but at the time of inclusion in this literature, some samples were not well informed about BMI and therefore did not perform subgroup analysis. Secondly, the synthesis of hormones is mainly in the testis. In addition to direct effects of SARS-CoV-2 virus on testis, fever, inflammation and other factors related to immune and stress response are also easily involved in the synthesis of testosterone. All the eligible studies included had fever symptoms, but there was no difference in fever grade, so subgroup analysis could not be performed. However, the diversity and uncertainty of clinical features, as well as whether various clinical manifestations will influence each other, also make subgroup analysis impossible. Finally, when we pooled the data by age group, the results showed that age was not the source of heterogeneity, suggesting that the effects of COVID-19 on male sex hormones were independent of age. Therefore, compared with non-reproductive age, male patients with fertility requirements and childbearing age should pay more attention to sex hormone levels, actively evaluate and consult, and prepare for the normalization of the epidemic.
There are also limitations to this systematic review. First of all, the sample size of the included studies varied greatly among studies, which may affect the accuracy of the meta-analysis results. Secondly, the search was limited to Chinese and English, and the literature may not be comprehensive enough to miss relevant studies in other languages. Finally, there were no long-term follow-up data from all studies to determine whether SARS-CoV-2 would have a long-term effect on sex hormones.
8 Conclusion
In summary, COVID-19 has a direct impact on sex hormones and may threaten male fertility, but the long-term effects on patients and whether they are reversible are unknown, and follow-up needs to be extended. More high-quality, large-sample studies need to be included in the future to further reveal the effects of COVID-19 on male sex hormones.
Data Availability
The authors vouch for the authenticity of the data and materials.
Abbreviations
- COVID-19:
-
Corona Virus Disease 2019
- BMI:
-
Body mass index
- LH:
-
Luteinizing hormone
- FSH:
-
Follicle stimulating hormone
- PRL:
-
Prolactin
- E2:
-
Estradiol
- ACE2:
-
Angiotensin converting enzyme 2
- TMPRSS2:
-
Type II transmembrane serine protease
References
Sun Bang Wu, Xueyan NM. Damage and mechanism of novel coronavirus to male reproduction. Chin J Androl. 2021;27(08):738–41. https://doi.org/10.13263/j.cnki.nja.2021.08.012.
Apaydin T, Sahin B, Dashdamirova S, Dincer Yazan C, Elbasan O, Ilgin C, et al. The association of free testosterone levels with coronavirus disease 2019. Andrology. 2022. https://doi.org/10.1111/andr.13152. (Epub 2022/01/08 PubMed PMID: 34994082).
Xu W, Lixiang Z, Liya A, Yingenzyme Z, Huan-Rui S, Ruo-peng Z. Effects of novel coronavirus disease 2019 (2019-NCOV) on reproductive systems of both sexes. Chin J Eugenics Genetics. 2021;29(06):884–7. https://doi.org/10.13404/j.cnki.cjbhh.20210927.019.
Cinislioglu AE, Cinislioglu N, Demirdogen SO, Sam E, Akkas F, Altay MS, et al. The relationship of serum testosterone levels with the clinical course and prognosis of COVID-19 disease in male patients: a prospective study. Andrology. 2022;10(1):24–33. https://doi.org/10.1111/andr.13081.
Enikeev D, Taratkin M, Morozov A, Petov V, Korolev D, Shpikina A, et al. Prospective two-arm study of the testicular function in patients with COVID-19. Andrology. 2022. https://doi.org/10.1111/andr.13159.
Guo TH, Sang MY, Bai S, Ma H, Wan YY, Jiang XH, et al. Semen parameters in men recovered from COVID-19. Asian J Androl. 2021;23(5):479–83. https://doi.org/10.4103/aja.aja_31_21. (Epub 2021/05/13 PubMed PMID: 33975987; PubMed Central PMCID: PMCPMC8451500).
Kadihasanoglu M, Aktas S, Yardimci E, Aral H, Kadioglu A. SARS-CoV-2 pneumonia affects male reproductive hormone levels: a prospective, cohort study. J Sex Med. 2021;18(2):256–64. https://doi.org/10.1016/j.jsxm.2020.11.007.
Ma L, Xie W, Li D, Shi L, Ye G, Mao Y, et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93(1):456–62. https://doi.org/10.1002/jmv.26259. (Epub 2020/07/06 PubMed PMID: 32621617; PubMed Central PMCID: PMCPMC7361404).
Salonia A, Pontillo M, Capogrosso P, Gregori S, Tassara M, Boeri L, et al. Severely low testosterone in males with COVID-19: a case-control study. Andrology. 2021;9(4):1043–52. https://doi.org/10.1111/andr.12993.
Temiz MZ, Dincer MM, Hacibey I, Yazar RO, Celik C, Kucuk SH, et al. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: a cross-sectional, pilot study. Andrologia. 2021. https://doi.org/10.1111/and.13912.
Xu H, Wang Z, Feng C, Yu W, Chen Y, Zeng X, et al. Effects of SARS-CoV-2 infection on male sex-related hormones in recovering patients. Andrology. 2021;9(1):107–14. https://doi.org/10.1111/andr.12942.
Wei TC, Huang WJ, Lin AT, Chen KK. The role of hormones on semen parameters in patients with idiopathic or varicocele-related oligoasthenoteratozoospermia (OAT) syndrome. J ChinMed Assoc JCMA. 2013;76(11):624–8. https://doi.org/10.1016/j.jcma.2013.07.005. (Epub 2013/08/13 PubMed PMID: 23933342).
Lotti F, Corona G, Maseroli E, Rossi M, Silverii A, Degl’innocenti S, et al. Clinical implications of measuring prolactin levels in males of infertile couples. Andrology. 2013;1(5):764–71. https://doi.org/10.1111/j.2047-2927.2013.00114.x. (Epub 2013/08/24 PubMed PMID: 23970454).
Zengliang L, Diping C, Guoli D. Correlation of sex hormone levels and sperm DNA integrity in infertile men. Sex Sci China. 2017;26(05):108–11. https://doi.org/10.3969/j.issn.1672-1993.2017.05.036.
Liu X, Chen Y, Tang W, Zhang L, Chen W, Yan Z, et al. Single-cell transcriptome analysis of the novel coronavirus (SARS-CoV-2) associated gene ACE2 expression in normal and non-obstructive azoospermia (NOA) human male testes. Sci China Life Sci. 2020;63(7):1006–15. https://doi.org/10.1007/s11427-020-1705-0. (Epub 2020/05/04 PubMed PMID: 32361911; PubMed Central PMCID: PMCPMC7195615).
Weijie Z. Defence mechanisms of testicular immune and considerations of male fertility impairment associated with SARS-CoV-2. Chin J Pathophysiol. 2020;36(07):1340–4. https://doi.org/10.3969/j.issn.1000-4718.2020.07.028.
Agolli A, Yukselen Z, Agolli O, Patel MH, Bhatt KP, Concepcion L, et al. SARS-CoV-2 effect on male infertility and its possible pathophysiological mechanisms. Discoveries (Craiova, Romania). 2021;9(2):e131. https://doi.org/10.15190/d.2021.10. (Epub 2021/11/25 PubMed PMID: 34816001; PubMed Central PMCID: PMCPMC8605861).
Giagulli VA, Guastamacchia E, Magrone T, Jirillo E, Lisco G, De Pergola G, et al. Worse progression of COVID-19 in men: Is testosterone a key factor? Andrology. 2021;9(1):53–64. https://doi.org/10.1111/andr.12836.
Pradhan A, Olsson PE. Sex differences in severity and mortality from COVID-19: are males more vulnerable? Biol Sex Differ. 2020;11(1):53. https://doi.org/10.1186/s13293-020-00330-7. (Epub 2020/09/20 PubMed PMID: 32948238; PubMed Central PMCID: PMCPMC7498997).
Navarra A, Albani E, Castellano S, Arruzzolo L, Levi-Setti PE. Coronavirus disease-19 infection: implications on male fertility and reproduction. Front Physiol. 2020. https://doi.org/10.3389/fphys.2020.574761. (Epub 2020/12/15 PubMed PMID: 33312128; PubMed Central PMCID: PMCPMC7704452).
Sengupta P, Dutta S. COVID-19 and hypogonadism: secondary immune responses rule-over endocrine mechanisms. Hum Fertil (Cambridge, England). 2021. https://doi.org/10.1080/14647273.2020.1867902. (Epub 2021/01/14 PubMed PMID: 33439057).
Abdel-Moneim A. COVID-19 pandemic and male fertility: clinical manifestations and pathogenic mechanisms. Biochemistry (Mosc). 2021;86(4):389–96. https://doi.org/10.1134/s0006297921040015. (Epub 2021/05/05 PubMed PMID: 33941061; PubMed Central PMCID: PMCPMC7978437).
Keskin MZ, Budak S, Aksoy EE, Yücel C, Karamazak S, Ilbey YO, et al. Investigation of the effect of body mass index (BMI) on semen parameters and male reproductive system hormone. Arch Ital Urol Androl. 2017;89(3):219–21. https://doi.org/10.4081/aiua.2017.3.219. (Epub 2017/10/04 PubMed PMID: 28969407).
Jiang G, Jun Z, Jing Z, Chunfang Y, Cuiru L. The impacts of male BMI on sperm parameters and sexual hormone levels: a meta-analysis. J Modern Urol. 2019;24(06):461–6. https://doi.org/10.3969/j.issn.1009-8291.2019.06.011.
Acknowledgements
None.
Funding
This study was funded by Youth Foundation of Sichuan Provincial Natural Science Foundation (project number: 2023NSFSC1802) and Chengdu University of Traditional Chinese Medicine “Xinglin Scholar” Disciplinary Talent Scientific Research Promotion Plan-Seedling Talent Special (project number: MPRC2023057).
Author information
Authors and Affiliations
Contributions
Conceptualization: XL, FY. Data collect: XL, LD. Data extraction: XL, FY, DC. Statistical analysis: XY, JL, MW. Writing-original draft: XL, MW. Writing-review and editing: XY, MW. Revise the drawing: DC. Revision of Writing: DC.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical Approval and Consent to Participate
None.
Consent for Publication
The authors agreed to publish.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lan, X., Chen, D., Wang, M. et al. The Effect of COVID-19 on Male Sex Hormones: A Meta-Analysis of Prospective Cohort Study. J Epidemiol Glob Health (2024). https://doi.org/10.1007/s44197-024-00203-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44197-024-00203-x