Abstract
The coronavirus disease 2019 (COVID-19) pandemic has led to significant changes in life and healthcare all over the world. Pregnant women and their newborns require extra attention due to the increased risk of adverse outcomes. Adverse pregnancy outcomes include intensive care unit (ICU) admission, pulmonary, cardiac, and renal impairment leading to mortality. Immaturity and variations of the neonatal immune system may be advantageous in responding to the virus. Neonates are at risk of vertical transmission and in-utero infection. Impaired intrauterine growth, prematurity, vertical transmission, and neonatal ICU admission are the most concerning issues. Data on maternal and neonatal outcomes should be interpreted cautiously due to study designs, patient characteristics, clinical variables, the effects of variants, and vaccination beyond the pandemic. Cesarean section, immediate separation of mother-infant dyads, isolation of neonates, and avoidance of breast milk were performed to reduce transmission risk at the beginning of the pandemic in the era of insufficient knowledge. Vertical transmission was found to be low with favorable short-term outcomes. Serious fetal and neonatal outcomes are not expected, according to growing evidence. Long-term effects may be associated with fetal programming. Knowledge and lessons from COVID-19 will be helpful for the next pandemic if it occurs.
Impact
-
Prenatal infection with SARS-CoV-2 is associated with adverse maternal and neonatal outcomes.
-
Our review includes the effects of COVID-19 on the fetus and neonates, transmission routes, placental effects, fetal and neonatal outcomes, and long-term effects on neonates.
-
There is a growing body of data and evidence about the COVID-19 pandemic. Knowledge and lessons from the pandemic will be helpful for the next pandemic if it happens.
Similar content being viewed by others
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has brought about significant changes in life and also healthcare worldwide. Since the beginning of the COVID-19 pandemic up to 21 May 2023, 688,961,970 cases have been reported worldwide, including 6,879,977 deaths, despite a decrease in the severity of illness.1 Coronaviruses are positive-sense, enveloped, single-stranded RNA viruses. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) binds to angiotensin-converting enzyme II (ACE2), such as SARS-CoV-1, and is responsible for COVID-19.2 The ACE2 receptor is primarily distributed through respiratory tract epithelium. Respiratory droplets are the primary transmission route, while airborne and contact transmission may also occur.3 After viral entry, Toll-like receptors lead to immune activation, resulting in a cytokine storm with both proinflammatory and antiinflammatory cytokines. Proinflammatory changes in mothers with COVID-19 can affect perinatal outcomes and influence neonatal inflammatory status, leading to high levels of plasma cytokines (interleukins, interferon, etc.), Natural killer (NK) cells, and T-regulatory cells that mimic maternal levels.4,5
The prevalence of COVID-19 in pregnant individuals diagnosed during labor and delivery has been reported to range between 3% and 20% from Türkiye and the United States of America (USA) in 2020.6,7 Pregnant women have an increased risk of acquiring the virus and experiencing adverse outcomes due to immune and cardiopulmonary changes including immunosuppression, an increased heart rate, stroke volume, oxygen consumption, diminished lung capacity, and an increased risk of thromboembolic disease.8,9,10 Inflammatory activity is heightened in the first and third trimester while it decreases and transitions into an anti-inflammatory stage in the second trimester.11
Adverse pregnancy outcomes of COVID-19 include admission to the intensive care unit (ICU), the need for endotracheal intubation, renal failure, death, and problems associated with cytokine storm, which can affect the placenta and fetus, leading to preterm birth, intrauterine growth retardation (IUGR), and low birth weight (LBW) (Fig. 1).12,13 Morbidity and mortality rates among pregnant women are higher than among non-pregnant women.14,15 In addition to these problems, vertical transmission is another important concern that can also affect both the fetus and neonates while intrapartum, and postpartum transmission is another important issue.16,17 The management of these fetus and neonates has evolved from strict precautions at the beginning of the pandemic to simpler measures as our knowledge of the clinical course of the disease and neonatal outcomes has increased. Variants of SARS-CoV-2 from Alpha to Omicron, COVID-19 during early or late gestation, the severity of the disease, and vaccination may all have an impact on maternal and fetal outcomes.18
Neonates have immature immune systems, frequent contact with family or healthcare caregivers, and various underlying conditions such as prematurity and LBW. The primary clinical presentations in neonates include fever, respiratory symptoms such as tachypnea, retractions, cough, poor feeding, and diarrhea.19,20,21
Neonates are less affected by COVID-19 than older children and adults, although they are more susceptible to infections than children.22 The clinical course of COVID-19 in neonates is usually mild, but long-term effects are not known. ACE2 receptor variations in children and adults may have an impact on resistance to SARS-CoV-2.23 The neutrophilic pathway in response to COVID-19, less adaptive immune development of neonates, and the absence of comorbidities and chronic low-grade inflammation seen in adults may be advantages in preventing hyperinflammation and cytokine storms in neonates.24
There is a growing body of literature and evidence about COVID-19, but it is challenging to evaluate them due to heterogeneity in study design, the risk of bias, selection of the patients, the definition of outcomes, and the effects of SARS-CoV-2 variants and global vaccination. We aim to evaluate prenatal exposure to COVID-19 and neonatal outcomes in the light of the current evidence.
Mother-to-child transmission
Placental transmission
SARS-CoV-2 enters the placenta with the aid of transmembrane serine protease 2 (TMPRSS2) by binding to ACE2.25,26 As gestation progresses, ACE2 and TMPRSS2 expression gradually decreases and is finally almost not expreesed in late gestation.27 Syncytial trophoblasts are relatively deficient in the first trimester and are associated with increased transmission of pathogens.28 Changes in the immune functions of the placenta throughout pregnancy may also affect the transmission of pathogens.29
The association of SARS-CoV-2 placental invasion and fetal involvement, such as lung and kidney in early pregnancy, may be suggested that SARS-CoV-2 can infect the fetus through the placenta, although late pregnancy placental transmission is less likely.30
Another placental transmission route involves the crossing of the placenta by monocytes and macrophages infected by SARS-CoV-2 and antibody/SARS-CoV-2 complex.31,32
The degree of maternal clinical severity, viremia, receptor expression in the mother-fetus, placentitis, placental defense, vaccination status, and the properties of variants are influencing factors for in-utero transmission.
Intrapartum transmission/vaginal ascending transmission
Intrapartum transmission may occur through contact with maternal genital mucosa, genital secretions, blood, urine, and feces during delivery. Theoretically, assisted vaginal delivery may carry additional risk. Although vaginal transmission through ascending infection during birth is a well-known route for early neonatal sepsis, the mode of delivery was reported not to be a risk factor because several studies showed that vaginal secretions of mothers with COVID-19 were SARS-CoV-2 negative, with no vertical transmission to their neonates.33,34
Ingestion or inhalation of amniotic fluid by the fetus is another possible transmission route. There are conflicting reports in regarding the presence of SARS-CoV-2 in amniotic fluid and cord blood.35,36 Rosen et al. performed amniocentesis on women with asymptomatic or mild disease in the first or second-trimester and found no polymerase chain reaction (PCR) positivity.37 This may also support the low risk of intrauterine transmission.
Breastfeeding transmission
Studies have shown that breast milk has no SARS-CoV-2 replication activity and have explained it by the absence of ACE2 expression in the breast tissue, although PCR positivity has been reported.38,39,40,41 The presence of RNA in the breast milk was not associated with infectivity.42 Breast milk is a unique source of nutrition for infants, but during the first wave of the pandemic breastfeeding was avoided due to the risk of transmission. However, breast milk contains SARS-CoV-2 IgG and IgA antibodies, proteomic changes, and boosts infants’ immunity.43 Maternal infection more than 2 weeks before delivery was associated with higher IgG transmission and the persistence of antibodies up to 6 months of age.44,45 Breastfeeding-related transmission after 72 h was not increased when hygiene measures such as surgical masks, hand hygiene, breast cleansing, and social distancing were practiced.46,47 Breastfeeding is now encouraged even in mothers with active infection, with precautions.48,49 Hygiene measures are the key factors in preventing breastfeeding-related and intrapartum transmission.
Placenta
Pathological process begins after ACE2 interaction leading to vasoconstriction, fibrosis, inflammation, thromboembolism, fibrin deposition, necrosis, and villitis, a condition known as placentitis.50 The placenta exhibits villous hyperplasia and mural hyperplasia due to immune reactions (complement activation and proinflammatory cytokines), and adverse perinatal outcomes have been attributed to chorioangiosis, fetal-side thrombosis, chorioamnionitis, and chronic villitis, that are associated with placental insufficiency.51,52 Viral antigens and nucleic acids have been detected in placentas with placentitis.53 These changes result in impaired delivery of oxygen and nutrients and may be associated with fetal hypoxia, stillbirth, LBW, and fetal distress.54,55,56 An international study evaluating placentas of 64 stillborns (mean gestational age 30 weeks) and four neonatal deaths (mean gestational age 30.8 weeks) reported placentitis in all patients and the detection of SARS-CoV-2 in 16 of 28 cases’ body specimens such as the nasopharynx and internal organs.57 Salvatore et al. investigated a cohort of 975 cases and found no association between the severity of placental inflammation and neonatal outcomes (preterm birth, small for gestational age, and neonatal ICU admission).58 However, the association between severe placentitis and fetal impairment should be considered, especially in first or second-trimester infections.
Maternal, fetal, and neonatal outcomes
The INTERCOVID study, a multinational cohort study that included 706 infected pregnancies from 18 countries, showed that maternal outcomes including mortality, ICU admission, preeclampsia, and eclampsia were increased.14,59 Preterm birth, LBW, cesarean section (C/S), and fetal distress were also more prevalent. Among the 586 neonates, 56 (9.5%) tested positive for SARS-CoV-2, this was associated with a higher rate of neonatal intensive care unit (NICU) admission, fever, gastrointestinal, and respiratory symptoms. The duration of in-utero exposure and C/S were associated with neonatal positivity, whereas breastfeeding, skin-to-skin contact, and rooming-in were not. The PAN-COVID study (n:8239), primarily recruited from the United Kingdom (UK), reported an increase in indicated preterm births but not IUGR, small for gestational age (SGA), and congenital anomalies.60 They also identified 80 out of 7993 (1%) neonates with SARS-CoV-2 PCR positivity.
Several studies from different countries have reported the outcomes of neonates born to mothers with COVID-19.2,19,61,62,63,64,65,66,67,68,69,70 Vertical transmission rates have been reported to range from 0% to 12%. The highest risk of infection for neonates born to mothers with COVID-19 occurs near the time of birth.70 Data from the Centers for Disease Control and Prevention (CDC) and the Perinatal COVID-19 registry revealed a PCR positivity rate of 5.6% to 13.6% among neonates in the first 3 days of life.71 Vertical transmission risk was increased by 2.4, 3.5, and 14 times in mothers with severe disease, the need for ICU care, and death, respectively.72 It can be speculated that the Omicron wave and increased vaccination have led to less vertical transmission due to reduced placental involvement and a milder clinical course.
Rates of prematurity, LBW, and NICU admission were reported to be mostly higher in neonates with suspected vertical transmission. At the beginning of the pandemic, most infants were kept in the NICU for isolation and close follow-up of an unknown clinical course. Admission criteria for the NICU changed during the pandemic from “as soon as possible after birth” to “any symptoms related to COVID-19”.73 Dumitrie et al. reported rooming-in and breastfeeding practices in New York, with a 2% vertical transmission rate and an increased risk of phototherapy in neonates born to mothers with severe disease.66 Chi et al. reviewed the outcomes of 105 neonates born to infected mothers in China, reporting prematurity, SGA, and PCR positivity rates of 23.8%, 11.25%, and 8.8%, respectively.36 They also noted that neonatal positivity was associated with a longer duration of maternal symptoms. Three neonates in two of the included studies had elevated levels of both IgM and IgG antibodies without PCR positivity after birth.74,75 This may be explained by maternal IgM transmission through placental inflammation, horizontal transmission during birth leading to an infection, or false positivity.
The Indian registry reported the outcomes of 1713 neonates, with 106 (8%) experiencing perinatal transmission.65 The majority of transmissed neonates were asymptomatic, while among symptomatic neonates (20%), prematurity and fetal distress were the main predisposing factors. A multicenter study from Spain reported no vertical transmission and a 46.3% NICU admission rate, with most admissions occuring for organizational reasons (75.6%).62 Another multicenter study from Türkiye at the beginning of the pandemic revealed rates of 3.3% (4/120) PCR positivity, 8.6% NICU admission, and 86.4% isolation room follow-up after delivery.19 Post-discharge follow-up of these infants revealed favorable outcomes.76,77 Neonates with SARS-CoV-2 PCR positivity were generally asymptomatic and had favorable outcomes, while symptomatic neonates usually had mild symptoms, mostly respiratory distress.20 The requirement for mechanical ventilation was reported between 0.4% and 1.2%.78 However, COVID-19-related respiratory distress was not definitively described in these neonates, and prematurity and C/S delivery, as reported in many reviews and meta-analyses, may be the main risk factors for developing respiratory distress and NICU admission.
Son et al. compared the prenatal outcomes of SARS-CoV-2 infected pregnant women (n:7432) with non-infected pregnant women (n:100635) between March and December 2020 in USA. The rates of preterm birth (8.5% vs 7.6%), stillbirth (0.4% vs 0.4%), SGA (6.4% vs 6.5%), large for gestational age (LGA) (7.7% vs 7.7%), hypertensive disorders of pregnancy (16.3% vs 15.8%) and C/S (31.2% vs 29.4%) were similar. Moreover, similar comparisons with the pre-pandemic period revealed no differences.79 An interesting finding of this study was that infection in the third trimester led to an almost two-fold increase in the risk of preterm birth compared to first or second-trimester infection. In contrast to the previous study, Canadian data with 6012 infected pregnancies at 28–37 weeks of gestation from March 2020 to October 2021 showed an increased risk of prematurity (11.05% vs 6.76%) even in milder disease, while rates of preeclampsia, stillbirth, and C/S were similar.80 More recent studies (n:12976 and 2708) from the USA reported an increased rate of preterm birth (11.5% vs 10.3% and 11.7% vs 7.7%) after infection in any trimester.81
The severity of COVID-19 during pregnancy may be associated with adverse fetal and neonatal outcomes such as fetal distress, preterm birth, C/S, IUGR, LBW, and SGA.82,83,84,85 Symptomatic mothers (n:90) had more preterm deliveries (16% vs 3%) and NICU admissions for their newborns (19% vs 2%) than asymptomatic mothers (n:59) in the first 3 months of the pandemic.86 A Spanish study, including 6872 infected mothers and their infants, reported an association between severe disease and an increased risk of C/S (90.9% vs 30.3%) and prematurity (72.7% vs 13.1%).87 Although Blitz et al. supported the increased risk of preterm delivery in symptomatic mothers, there were also non-supportive studies.88,89,90,91 It should be noted that iatrogenic preterm delivery and the indication for C/S are associated with the severity of infection.92,93
Pregnant women with co-morbid diseases such as obesity, hypertension, respiratory disease, and Black ethnicity are prone to severe COVID-19.94 A meta-analysis evaluating being symptomatic or severe COVID-19 showed higher odds of preterm birth, C/S, LBW, and NICU admission.94,95 Both meta-analyses did not report an increased risk of vertical transmission. COVID-19 seems to be an additional risk factor for co-morbid diseases, leading to worse perinatal outcomes.
The timing of infection throughout pregnancy may have an effect on outcomes. Sahin et al. reported the outcomes of 311, 433, and 672 pregnant women in the first, second, and third trimesters. Rates of mild, severe/critical COVID‐19, and hospitalization were highest in the first, second, and third trimesters, respectively.96 Pregnant women with severe infection (n:616 of 4436, 13.9%) had increased rates of pre-labor C/S, preterm birth, stillbirth, and NICU admission, while discharged women (n:112) had a chance to deliver >36 weeks of gestation.97 This study also reported a decreased risk of adverse neonatal outcomes with an increased time from diagnosis to birth. UK data showed that delivery ≥37 weeks of gestation was not associated with adverse neonatal outcomes.93 Infections in the first or second-trimester should be given attention because first and second-trimester infections especially in severe disease, were associated with fetal/neonatal death and preterm birth but not with SGA, major congenital malformation, or hypertensive diseases of pregnancy compared to no SARS-CoV-2 infected pregnancies.30,98,99 Alcover et al. reported that stillbirths/late miscarriages occurred 6–13 days after the beginning of symptoms or confirmation of infection, and 66% of fetuses had organ involvement with SARS-CoV-2.100 Regan et al. reported that 6.4%, 19.3%, and 74.2% of infections were diagnosed in the first, second, and third trimesters of 2655 pregnant women and found an increased adjusted hazard ratio for IUGR, C/S, and preterm birth in any trimester.101 Reports should be carefully interpreted because there are too many variables, such as insufficient data at the beginning of the pandemic, the fear of vertical transmission, indications for the delivery route, the developing of variants, timing of infection during pregnancy, and different methodologies and outcomes of studies.
Meta-analyses
Smith et al. reported increased adverse maternal outcomes at any gestational age in their meta-analysis (7 studies, 7637 neonates).13 They also reported that neonatal mortality risk was not increased despite increased NICU admission. Preterm birth was found to be increased, but the effect of spontaneous and iatrogenic preterm birth, especially at the beginning of the pandemic was not distinguished. Similar to this meta-analysis, the INTERCOVID study did not report the association between maternal SARS-CoV-2 infection and SGA. Stillbirth risk was not found to be increased, in contrast to UK data, which included 340,000 pregnancies, and a living systematic review and meta-analysis by Allotey et al.93,102
Panda et al. reported the outcomes of 3551 neonates born to infected mothers in India and compared them with an Indian database.103 They found an increase in the rate of C/S, prematurity, LBW, and also lower mortality with good clinical outcomes for neonates. Admission to the NICU was attributed to asphyxia, sepsis-like conditions, and prematurity. It should be noted that some neonatal SARS-CoV-2 positivity was diagnosed after 72 h.
Mark et al. reviewed 1922 infected pregnant women, mostly in the third trimester, and 1361 neonates mostly from the USA and China.82 Immediately separation from the mother after birth was performed in 67% of neonates. The most common symptom of neonates was respiratory distress (n:126, 21%) while SARS-CoV-2 positive (n:61) and negative (n:1269) neonates had prematurity rates of 55% vs 27% and respiratory distress rates of 69% and 17%, respectively, leading to a greater need for mechanical ventilation treatment. Short-term follow-up through telephone or clinic visits revealed good outcomes.47
Di Toro et al. reviewed the outcomes of 1100 infected pregnancies from China, Europe, and North America.73 The rates of preterm birth, emergency C/S, preeclampsia, preterm premature rupture of membranes (PPROM), and neonatal respiratory distress were 23%, 7%, 7%, 10%, and 4%, respectively. Three neonates died without COVID-19 relationship. While 19 neonates tested positive for PCR, 6 of them had RNA detection in the first 12 h of life, the others were tested 12–48 h after birth. Among these neonates, 4 had symptoms such as lethargy, fever, respiratory distress, and gastrointestinal symptoms.
Huntly et al. found similar rates of fetal death, neonatal death, preterm birth, NICU admission, and SGA in neonates of 728 infected and 3836 non-infected mothers.104 In contrast to the previous meta-analysis, a living systematic review and meta-analysis updated up to April 2021 revealed higher odds of preterm birth, stillbirth, neonatal death, fetal distress, and NICU admission in 293,152 pregnant and recently pregnant women with COVID-19.102
A recent systematic review and meta-analysis included 1,843,278 pregnancies up to September 2022.105 Pregnant individuals with COVID-19 experienced higher rates of preterm birth, PPROM, prematurity, fetal distress, gestational hypertensive diseases, gestational diabetes, stillbirth, and maternal mortality than pregnant individuals without COVID-19. The admission of neonates to the NICU was found to be increased, but it should be noted that some studies revealed unchanged rates, which may be associated with the different timelines during the pandemic. Neonates with SARS-CoV-2 positivity were reported in 32 studies, with a rate of 2%. The study also found that pregnancy loss and SARS-CoV-2 positive neonates were more prevalent in lower-middle-income countries than higher-income countries although the income of the country was not found to be associated with preterm birth and NICU admission.105,106
In an overview of systematic reviews, preterm births were reported between 14.3% and 63.8%, while SARS-CoV-2 infection indicated preterm birth rate was 49.6%.107 LBW was reported as 7.8% after including data from the largest reviews. Abnormal Apgar scores at 5 min were calculated to be 2.2%, while neonatal asphyxia rates ranged from 0.6 to less than 13%. Admission to the NICU rates were highly variable, ranging from 3.1% to 76.9%, with 27.3% in the largest sample. Neonatal SARS-CoV-2 PCR positivity rates ranged between 1.6% and 10%, with 2.5% (n:19/751) and 5.5% (n:58/1048) in the two largest series. The authors also evaluated the tested samples, such as placenta, amniotic fluid, and umbilical cord blood, for SARS-CoV-2 PCR and the most striking result was that almost all neonates who tested positive in these samples had no nasopharyngeal PCR positivity. Table 1 shows the results of some current meta-analyses.13,21,73,78,82,93,95,102,103,106,107,108,109,110,111,112,113,114 Stillbirth was reported in up to 4% of pregnancies, with an increased risk. Fetal and neonatal deaths may be associated with direct infection, timing of infection, maternal illness leading to insufficient placental circulation, and placental involvement and insufficiency. However, results should be evaluated, considering the effects of variants, vaccination, and the power of the studies.
Cesarean section rates increased due to fear of vertical transmission at the beginning of the pandemic and gradually decreased as our knowledge increased, with an indication for moderate and severe illness.115 Nowadays, the choice of delivery route is recommended based on obstetric indications, and severe maternal illness with respiratory compromise seems to be a major indication for C/S.116
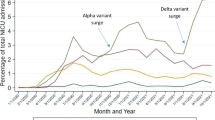
Preterm birth and LBW were found to be increased in SARS-CoV-2 infected pregnancies in many studies and were associated with moderate and severe illness.115 Preterm birth rates were also affected by waves and were reported as 9.9%, 20.3%, and 18.4% in New York City at the beginning of the pandemic, pre-Delta, and Omicron waves, respectively.117 Vaccination led to a decreased preterm birth rate (5.1% vs 9.1%), but simultaneous timing of vaccination and the mild clinical outcomes of the Omicron wave should be kept in mind.118
Fetal effects
Fetal effects may be expected due to the direct or indirect effects of SARS-CoV-2 such as maternal hyperthermia, placental involvement, or cytokine storm, especially in the 1st weeks of gestation. Fetal loss due to hydrops fetalis diagnosed at the 13th week of gestation after COVID-19 at the 8th week of gestation was reported with SARS-CoV-2 positivity in the placenta, amniotic fluid, and fetal membranes.119 Fetal lung growth was found to be impaired due to third trimester infection according to fetal MRI, although it was not clearly explained.120 Another fetal MRI study (n:38), performed at a median of 27th weeks of gestation, revealed cerebrovascular lesions in two fetuses and nonspecific signal hypointensities in the liver of two fetuses, with placental involvement in all fetuses.121
Various clinical diagnoses, such as fetal myocarditis, tachycardia, calcifications of fetal bowel and bladder, VACTERL association, fetal periventricular leukomalacia, cerebral venous thrombosis, cerebral ischemic lesions, neonatal necrotizing enterocolitis, and infantile immune thrombocytopenia, have attributed to COVID-19 during pregnancy.122,123,124,125,126,127,128,129 The International Registry of Coronavirus Exposure in Pregnancy showed that first-trimester exposure was not associated with a specific pattern of malformations.130 Vaccination was reported to be associated with a reduced rate of major congenital anomalies (2.4% vs 3%), but further studies are needed.118 Reduced fetal thymus size, which may affect long-term immunologic response, was reported due to inflammatory reactions associated with mild or moderate COVID-19.131 It is difficult to establish a clear relationship between COVID-19 and these conditions.
Multisystem inflammatory syndrome in neonates (MIS-N)
Multisystem inflammatory syndrome in children (MIS-C) is characterized by the involvement of at least two organ systems and elevated proinflammatory and antiinflammatory markers after SARS-CoV-2 infection.132 Clinical manifestations include fever, cardiovascular system involvement (shock, left ventricular dysfunction, elevated cardiac enzymes, coronary artery abnormalities, myocarditis, pericarditis), gastrointestinal system symptoms (nausea, vomiting, diarrhea similar to inflammatory bowel disease, necrotizing enterocolitis), and mucocutaneous symptoms.133 No uniform diagnostic criteria for multisystem inflammatory syndrome in neonates (MIS-N) have been developed yet, and neonates experience many similar clinical problems, such as sepsis, necrotizing enterocolitis, and respiratory distress syndrome.134,135 Inflammatory markers, including D-dimer, C-reactive protein, procalcitonin, lactate dehydrogenase, and ferritin are increased.136
In a systemic review, outcomes of 94 neonates with MIS-N were reported.136 The main symptoms were due to cardiac and respiratory involvement, and fever was predominantly present in the first week of life. MIS-N incidence in neonates with vertical transmission was nearly 0%, but it was 8% in symptomatic neonates. Both preterm and term infants were affected, the mortality rate was 9%. Systemic corticosteroids (methylprednisolone, dexamethasone) and immunoglobulins in addition to supportive care, are mostly used successfully based on studies in children and adults.17,137,138 Aspirin and low molecular weight heparin for thrombosis, and anakinra for severe/refractory cases, can also be used.134 Neurodevelopmental and cardiac follow-up should be performed.
Long-term effects
It is well-known that inadequate bonding has long-term consequences.139 Bonding can be impaired due to the separation of mother-infant dyads because of active SARS-CoV-2 infection of either of them, and perinatal infection, to prevent vertical or horizontal transmission.140,141
A systematic review of perinatal mental health outcomes in the first year of the pandemic showed increased psychological symptoms, especially depression and anxiety, in pregnant and postpartum women.142 Maternal psychosocial stress leading to disturbances in the hypothalamic-pituitary-adrenal axis can affect fetal maturation and development, with increased hormonal changes including cortisol and cytokine levels.143,144
Maternal psychosocial stress, impaired vaginal and intestinal microbiome, and impaired fetal-gut axis are linked to physiological alteration in the fetal brain, mainly fetal microglia, and neurobehavioral diseases, such as cognitive and behavioral dysfunction, schizophrenia, autism, depression, eating disorders, learning, and mood disorders.145,146,147 Brain tissue has ACE2 and is a target of SARS-Cov-2.148 Maternal immune activation and placental dysfunction may have a role in neurological impairment.149 Rajagopalan et al. evaluated fetal brain development with MRI of 45 fetuses at a mean of 31.5 weeks and found that increased maternal perception of pandemic-related stress correlated with reduced global fetal brain temporal function and functional connectivity.150 Both impaired and favorable results on neurodevelopmental outcomes were reported from different countries.151,152 Motor developmental delay and socioemotional alterations were found in infants with maternal depression.153 Ayed et al. pointed out that first or second-trimester infections carry a higher developmental risk than third trimester infections.151,154,155 In contrast to a previous study, Edlow et al. reported increased neurodevelopmental diseases at 12 months, especially in infants exposed to the virus in the third trimester.156 Transient early fine motor abnormalities were reported in 100 infants born to infected mothers with little or no risks of neurodevelopmental problems.157 An absence of fidgety movements between 3–5 months in 28 infants was found to be associated with increased neurological disorder risk.158 Neuroimaging of infants may show white matter abnormalities and vascular lesions, but further studies are needed to establish an association.149 The long-term consequences of in-utero exposure to the 1918 Influenza pandemic included increased depression, diabetes, ischemic heart disease, renal disease, and mortality after age 50, and also neurodevelopmental problems such as autism, anxiety, and schizophrenia that may be similar with fetal programming of IUGR and LBW infants.159,160,161 Differential gene expression and epigenomic methylation of DNA and histones may be markers for long-term effects.162,163 Lower birth weight and accelerated weight gain in the first year of life in infants with prenatal COVID-19 were reported, and the authors emphasized follow-up for cardiometabolic problems.164
Conclusion
Knowledge of COVID-19’s effects on fetal and neonatal outcomes has been evolving, much like our understanding of its clinical features. Management of infected mothers and their newborns is better understood during the pandemic and is continuously evolving. Increased rates of C/S, separation of mother-infant dyads, and avoiding breast milk were precautions taken to decrease vertical transmission at the beginning of the pandemic, but this is no longer the case. Impaired intrauterine growth and problems related to prematurity, vertical transmission, and NICU admission were the most investigated morbidities. Data showed that serious fetal and neonatal outcomes are not typically expected due to the direct effect of the virus in the era of the global impact of COVID-19. Vertical transmission seems to be low with favorable short-term outcomes but long-term effects should be evaluated. Low and middle-income countries may experience impaired outcomes due to worse maternal and neonatal healthcare status and higher rates of COVID-19 transmission. Results of studies and meta-analyses should be interpreted cautiously due to variations in study designs, patient characteristics, clinical variables, differentiation of viral effects, and vaccination beyond the pandemic, which can lead to contradictory results, including an insufficient understanding of cause and effect relationships. Knowledge and lessons from COVID-19 will be helpful for the next pandemic if it occurs.
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Covid-19 Coronavirus Outbreak, https://www.worldometers.info/coronavirus/ (2021).
Manti, S. et al. Effects of vertical transmission of respiratory viruses to the offspring. Front. Immunol. 13, 853009 (2022).
Sankaran, D., Nakra, N., Cheema, R., Blumberg, D. & Lakshminrusimha, S. Perinatal Sars-Cov-2 infection and neonatal Covid-19: a 2021 update. Neoreviews 22, e284–e295 (2021).
Gee, S. et al. The legacy of maternal Sars-Cov-2 infection on the immunology of the neonate. Nat. Immunol. 22, 1490–1502 (2021).
Rubio, R. et al. Maternal and neonatal immune response to Sars-Cov-2, IgG transplacental transfer and cytokine profile. Front. Immunol. 13, 999136 (2022).
Jamieson, D. J. & Rasmussen, S. A. An update on Covid-19 and pregnancy. Am. J. Obstet. Gynecol. 226, 177–186 (2022).
Tanacan, A. et al. The rate of Sars-Cov-2 Positivity in asymptomatic pregnant women admitted to hospital for delivery: experience of a pandemic center in Turkey. Eur. J. Obstet. Gynecol. Reprod. Biol. 253, 31–34 (2020).
Jamieson, D. J. et al. H1n1 2009 influenza virus infection during pregnancy in the USA. Lancet 374, 451–458 (2009).
Zambrano, L. D. et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed Sars-Cov-2 infection by pregnancy status—United States, January 22-October 3, 2020. MMWR Morb. Mortal. Wkly Rep. 69, 1641–1647 (2020).
Lokken, E. M. et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome Coronavirus 2 infection in Washington state. Am. J. Obstet. Gynecol. 225, 77.e1–77.e14 (2021).
Liu, H. et al. Why are pregnant women susceptible to Covid-19? An immunological viewpoint. J. Reprod. Immunol. 139, 103122 (2020).
Chowdhury, S. et al. Covid-19 and pregnancy. Discoveries (Craiova) 10, e147 (2022).
Smith, E. R. et al. Adverse maternal, fetal, and newborn outcomes among pregnant women with Sars-Cov-2 infection: an individual participant data meta-analysis. BMJ Glob. Health 8, e009495 (2023).
Villar, J. et al. Maternal and neonatal morbidity and mortality among pregnant women with and without Covid-19 infection: the intercovid multinational cohort study. JAMA Pediatr. 175, 817–826 (2021).
Kim, Y. K. & Kim, E. H. Pregnancy and Covid-19: past, present and future. Obstet. Gynecol. Sci. 66, 149–160 (2023).
Wang, J. & Dong, W. Covid-19: the possibility, ways, mechanisms, and interruptions of mother-to-child transmission. Arch. Gynecol. Obstet. 307, 1687–1696 (2023).
Ryan, L. et al. Neonates and Covid-19: state of the art : neonatal sepsis series. Pediatr. Res. 91, 432–439 (2022).
Sahin, D. et al. Comparison of clinical features and perinatal outcomes between pre-variant and post-variant periods in pregnant women with Sars-Cov-2: analysis of 1935 cases. Arch. Gynecol. Obstet. 306, 1939–1948 (2022).
Oncel, M. Y. et al. A multicenter study on epidemiological and clinical characteristics of 125 newborns born to women infected with Covid-19 by Turkish Neonatal Society. Eur. J. Pediatr. 180, 733–742 (2021).
Akin, I. M. et al. Epidemiologic and clinical characteristics of neonates with late-onset Covid-19: 1-year data of Turkish Neonatal Society. Eur. J. Pediatr. 181, 1933–1942 (2022).
Bellos, I., Pandita, A. & Panza, R. Maternal and perinatal outcomes in pregnant women infected by Sars-Cov-2: a meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 256, 194–204 (2021).
Dong, Y. et al. Epidemiology of Covid-19 among children in China. Pediatrics 145, e20200702 (2020).
Yoon, H. E. et al. Age-associated changes in the vascular renin-angiotensin system in mice. Oxid. Med. Cell Longev. 2016, 6731093 (2016).
Zimmermann, P. & Curtis, N. Why is Covid-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of Sars-Cov-2 infections. Arch. Dis. Child 106, 429–439 (2021).
Komine-Aizawa, S., Takada, K. & Hayakawa, S. Placental barrier against Covid-19. Placenta 99, 45–49 (2020).
Hartenian, E. et al. The molecular virology of coronaviruses. J. Biol. Chem. 295, 12910–12934 (2020).
Bloise, E. et al. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am. J. Obstet. Gynecol. 224, 298.e1–298.e8 (2021).
Jones, C. J., Harris, L. K., Whittingham, J., Aplin, J. D. & Mayhew, T. M. A re-appraisal of the morphophenotype and basal lamina coverage of cytotrophoblasts in human term placenta. Placenta 29, 215–219 (2008).
Yockey, L. J., Lucas, C. & Iwasaki, A. Contributions of maternal and fetal antiviral immunity in congenital disease. Science 368, 608–612 (2020).
Valdespino-Vazquez, M. Y. et al. Fetal and placental infection with Sars-Cov-2 in early pregnancy. J. Med. Virol. 93, 4480–4487 (2021).
Jafarzadeh, A., Chauhan, P., Saha, B., Jafarzadeh, S. & Nemati, M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in Covid-19: lessons from Sars and Mers, and potential therapeutic interventions. Life Sci. 257, 118102 (2020).
Ulrich, H., Pillat, M. M., Tarnok, A. & Dengue Fever, Covid-19 (Sars-Cov-2), and Antibody-Dependent Enhancement (Ade): a perspective. Cytom. A 97, 662–667 (2020).
Qiu, L. et al. Sars-Cov-2 is not detectable in the vaginal fluid of women with severe Covid-19 infection. Clin. Infect. Dis. 71, 813–817 (2020).
Lopian, M. et al. Safety of vaginal delivery in women infected with Covid-19. Pediatr. Neonatol. 62, 90–96 (2021).
Chen, H. et al. Clinical characteristics and intrauterine vertical transmission potential of Covid-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 395, 809–815 (2020).
Chi, H. et al. Clinical features of neonates born to mothers with coronavirus disease-2019: a systematic review of 105 neonates. J. Microbiol Immunol. Infect. 54, 69–76 (2021).
Rosen, H. et al. Fetal and perinatal outcome following first and second trimester Covid-19 infection: evidence from a prospective cohort study. J. Clin. Med. 10, 2152 (2021).
Hikmet, F. et al. The protein expression profile of Ace2 in human tissues. Mol. Syst. Biol. 16, e9610 (2020).
Oliveira, K. F., Oliveira, J. F., Wernet, M., Paschoini, M. C. & Ruiz, M. T. Vertical transmission and Covid-19: a scoping review. Rev. Bras. Enferm. 74, e20200849 (2021).
Chambers, C. et al. Evaluation for Sars-Cov-2 in breast milk from 18 infected women. JAMA 324, 1347–1348 (2020).
Sahin, D. et al. A pandemic center’s experience of managing pregnant women with Covid-19 infection in Turkey: a prospective cohort study. Int J. Gynaecol. Obstet. 151, 74–82 (2020).
Krogstad, P. et al. No infectious Sars-Cov-2 in breast milk from a cohort of 110 lactating women. Pediatr. Res. 92, 1140–1145 (2022).
Guo, J. et al. Proteomic analysis of human milk reveals nutritional and immune benefits in the colostrum from mothers with Covid-19. Nutrients 14, 2513 (2022).
Song, D. et al. Passive and active immunity in infants born to mothers with Sars-Cov-2 infection during pregnancy: prospective cohort study. BMJ Open 11, e053036 (2021).
Wang, X. et al. Dynamic changes of acquired maternal Sars-Cov-2 IgG in infants. Sci. Rep. 11, 8021 (2021).
Raschetti, R. et al. Synthesis and Systematic review of reported neonatal Sars-Cov-2 infections. Nat. Commun. 11, 5164 (2020).
Salvatore, C. M. et al. Neonatal management and outcomes during the Covid-19 pandemic: an observation cohort study. Lancet Child Adolesc. Health 4, 721–727 (2020).
Cdc. Coronavirus Disease (Covid-19) and Breastfeeding, https://www.cdc.gov/breastfeeding/breastfeeding-special-circumstances/maternal-or-infant-illnesses/covid-19-and-breastfeeding.html (2023).
Koc, E. & Dilli, D. How does Covid-19 affect maternal and neonatal outcomes? J. Perinat. Med. 51, 277–283 (2023).
Watkins, J. C., Torous, V. F. & Roberts, D. J. Defining severe acute respiratory syndrome coronavirus 2 (Sars-Cov-2) placentitis. Arch. Pathol. Lab. Med. 145, 1341–1349 (2021).
Figueiro-Filho, E. A., Hobson, S. R., Farine, D. & Yudin, M. H. Highly expressed Ace-2 receptors during pregnancy: a protective factor for Sars-Cov-2 infection? Med. Hypotheses 153, 110641 (2021).
Sharps, M. C. et al. A structured review of placental morphology and histopathological lesions associated with Sars-Cov-2 infection. Placenta 101, 13–29 (2020).
Schwartz, D. A., Mulkey, S. B. & Roberts, D. J. Sars-Cov-2 placentitis, stillbirth, and maternal Covid-19 vaccination: clinical-pathologic correlations. Am. J. Obstet. Gynecol. 228, 261–269 (2023).
Zaigham, M. et al. Clinical-pathological features in placentas of pregnancies with Sars-Cov-2 infection and adverse outcome: case series with and without congenital transmission. BJOG 129, 1361–1374 (2022).
Bouachba, A. et al. Placental lesions and Sars-Cov-2 infection: diffuse placenta damage associated to poor fetal outcome. Placenta 112, 97–104 (2021).
Schwartz, D. A. et al. Chronic histiocytic intervillositis with trophoblast necrosis is a risk factor associated with placental infection from coronavirus disease 2019 (Covid-19) and intrauterine maternal-fetal severe acute respiratory syndrome coronavirus 2 (Sars-Cov-2) transmission in live-born and stillborn infants. Arch. Pathol. Lab. Med. 145, 517–528 (2021).
Schwartz, D. A. et al. Placental tissue destruction and insufficiency from Covid-19 causes stillbirth and neonatal death from hypoxic-ischemic injury. Arch. Pathol. Lab. Med. 146, 660–676 (2022).
Salvatore, M. A. et al. Placental characteristics of a large Italian cohort of Sars-Cov-2-positive pregnant women. Microorganisms 10, 1435 (2022).
Giuliani, F. et al. Effects of prenatal exposure to maternal Covid-19 and perinatal care on neonatal outcome: results from the intercovid multinational cohort study. Am. J. Obstet. Gynecol. 227, 488.e1–488.e17 (2022).
Mullins, E. et al. Pregnancy and neonatal outcomes of Covid-19: the pan-covid study. Eur. J. Obstet. Gynecol. Reprod. Biol. 276, 161–167 (2022).
Vigil-Vazquez, S. et al. Impact of gestational Covid-19 on neonatal outcomes: is vertical infection possible? Pediatr. Infect. Dis. J. 41, 466–472 (2022).
Marin Gabriel, M. A. et al. Maternal, perinatal and neonatal outcomes with Covid-19: a multicenter study of 242 pregnancies and their 248 infant newborns during their first month of life. Pediatr. Infect. Dis. J. 39, e393–e397 (2020).
Nayak, M. K. et al. Neonatal outcomes of pregnant women with Covid-19 in a developing country setup. Pediatr. Neonatol. 62, 499–505 (2021).
Angelidou, A. et al. Association of maternal perinatal Sars-Cov-2 infection with neonatal outcomes during the Covid-19 pandemic in Massachusetts. JAMA Netw. Open 4, e217523 (2021).
More, K. et al. Outcomes of neonates born to mothers with coronavirus disease 2019 (Covid-19)—National Neonatology Forum (Nnf) India Covid-19 registry. Indian Pediatr. 58, 525–531 (2021).
Dumitriu, D. et al. Outcomes of neonates born to mothers with severe acute respiratory syndrome coronavirus 2 infection at a large medical center in New York city. JAMA Pediatr. 175, 157–167 (2021).
Kumar, C. et al. Perinatal transmission and outcomes of Sars-Cov-2 infection. Indian J. Pediatr. 89, 1123–1125 (2022).
Heidary, Z. et al. Maternal and neonatal complications, outcomes and possibility of vertical transmission in iranian women with Covid-19. Arch. Iran. Med. 24, 713–721 (2021).
Al-Lawama, M. et al. Perinatal transmission and clinical outcomes of neonates born to Sars-Cov-2-positive mothers. J. Clin. Med. Res. 13, 420–424 (2021).
Hudak, M. L. et al. Maternal and newborn hospital outcomes of perinatal Sars-Cov-2 infection: a national registry. Pediatrics 151, e2022059595 (2023).
American Academy of Pediatrics. Faqs: Management of Infants Born to Mothers with Suspected or Confirmed Covid-19 (Last Updated:11/10/2022), https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/faqs-management-of-infants-born-to-covid-19-mothers/.
Allotey, J. et al. Sars-Cov-2 positivity in offspring and timing of mother-to-child transmission: living systematic review and meta-analysis. BMJ 376, e067696 (2022).
Di Toro, F. et al. Impact of Covid-19 on maternal and neonatal outcomes: a systematic review and meta-analysis. Clin. Microbiol Infect. 27, 36–46 (2021).
Dong, L. et al. Possible vertical transmission of Sars-Cov-2 from an infected mother to her newborn. JAMA 323, 1846–1848 (2020).
Zeng, H. et al. Antibodies in infants born to mothers with Covid-19 pneumonia. JAMA 323, 1848–1849 (2020).
Flannery, D. D. et al. Perinatal Covid-19 maternal and neonatal outcomes at two academic birth hospitals. J. Perinatol. 42, 1338–1345 (2022).
Capozza, M. et al. Perinatal transmission and outcome of neonates born to Sars-Cov-2-positive mothers: the experience of 2 highly endemic Italian regions. Neonatology 118, 665–671 (2021).
Vergara-Merino, L. et al. Maternal and perinatal outcomes related to Covid-19 and pregnancy: an overview of systematic reviews. Acta Obstet. Gynecol. Scand. 100, 1200–1218 (2021).
Son, M. et al. Coronavirus disease 2019 (Covid-19) pandemic and pregnancy outcomes in a U.S. population. Obstet. Gynecol. 138, 542–551 (2021).
McClymont, E. et al. Association of Sars-Cov-2 infection during pregnancy with maternal and perinatal outcomes. JAMA 327, 1983–1991 (2022).
Doyle, T. J. et al. Maternal and perinatal outcomes associated with severe acute respiratory syndrome coronavirus 2 (Sars-Cov-2) infection during pregnancy, Florida, 2020–2021: a retrospective cohort study. Clin. Infect. Dis. 75, S308–S316 (2022).
Mark, E. G. et al. Coronavirus disease 2019 in pregnancy and outcomes among pregnant women and neonates: a literature review. Pediatr. Infect. Dis. J. 40, 473–478 (2021).
Mullins, E., Evans, D., Viner, R. M., O’Brien, P. & Morris, E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet. Gynecol. 55, 586–592 (2020).
Hamidi, O. P. et al. Adverse perinatal outcomes in pregnancies affected by severe Covid-19 infection. AJOG Glob. Rep. 2, 100118 (2022).
Vouga, M. et al. Maternal Outcomes and risk factors for Covid-19 severity among pregnant women. Sci. Rep. 11, 13898 (2021).
Verma, S. et al. Outcomes of maternal-newborn dyads after maternal Sars-Cov-2. Pediatrics 146, e2020005637 (2020).
Sanchez-Luna, M. et al. Neonates born to mothers with COVID-19: data from the Spanish society of neonatology registry. Pediatrics 147, e2020015065 (2021).
Blitz, M. J. et al. Preterm birth among women with and without severe acute respiratory syndrome coronavirus 2 infection. Acta Obstet. Gynecol. Scand. 100, 2253–2259 (2021).
Cruz-Lemini, M. et al. Obstetric outcomes of Sars-Cov-2 infection in asymptomatic pregnant women. Viruses 13, 112 (2021).
Babic, I. et al. Covid-19 pandemic and its impact on perinatal outcomes between symptomatic and asymptomatic women. Obstet. Gynecol. Int. 2022, 1756266 (2022).
Martinez-Varea, A. et al. Comparison of maternal-fetal outcomes among unvaccinated and vaccinated pregnant women with Covid-19. J. Pers. Med. 12, 2008 (2022).
Metz, T. D. et al. Association of Sars-Cov-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA 327, 748–759 (2022).
Gurol-Urganci, I. et al. Maternal and perinatal outcomes of pregnant women with Sars-Cov-2 infection at the time of birth in England: national cohort study. Am. J. Obstet. Gynecol. 225, 522.e1–522.e11 (2021).
Khan, D. S. A. et al. Differences in pregnancy and perinatal outcomes among symptomatic versus asymptomatic Covid-19-infected pregnant women: a systematic review and meta-analysis. BMC Pregnancy Childbirth 21, 801 (2021).
Lassi, Z. S. et al. A systematic review and meta-analysis of data on pregnant women with confirmed Covid-19: clinical presentation, and pregnancy and perinatal outcomes based on Covid-19 severity. J. Glob. Health 11, 05018 (2021).
Sahin, D. et al. Management of pregnant women with Covid-19: a tertiary pandemic center experience on 1416 cases. J. Med. Virol. 94, 1074–1084 (2022).
Vousden, N. et al. Management and implications of severe Covid-19 in pregnancy in the UK: data from the UK obstetric surveillance system national cohort. Acta Obstet. Gynecol. Scand. 101, 461–470 (2022).
Hughes, B. L. et al. First- or second-trimester Sars-Cov-2 infection and subsequent pregnancy outcomes. Am. J. Obstet. Gynecol. 228, 226.e1–226.e9 (2023).
Piekos, S. N. et al. The effect of maternal Sars-Cov-2 infection timing on birth outcomes: a retrospective multicentre cohort study. Lancet Digit Health 4, e95–e104 (2022).
Alcover, N. et al. Systematic review and synthesis of stillbirths and late miscarriages following Sars-Cov-2 infections. Am. J. Obstet. Gynecol. 229, 118–128 (2023).
Regan, A. K., Arah, O. A., Fell, D. B. & Sullivan, S. G. Sars-Cov-2 infection during pregnancy and associated perinatal health outcomes: a national us cohort study. J. Infect. Dis. 225, 759–767 (2022).
Allotey, J. et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 370, m3320 (2020).
Panda, S. K., Mishra, A. & Pathak, M. Clinical outcome of neonates born to Sars-Cov-2 positive mothers in India: a systematic review and meta-analysis. Cureus 14, e22958 (2022).
Huntley, B. J. F. et al. Adverse pregnancy outcomes among individuals with and without severe acute respiratory syndrome coronavirus 2 (Sars-Cov-2): a systematic review and meta-analysis. Obstet. Gynecol. 137, 585–596 (2021).
Simbar, M., Nazarpour, S. & Sheidaei, A. Evaluation of pregnancy outcomes in mothers with Covid-19 infection: a systematic review and meta-analysis. J. Obstet. Gynaecol. 43, 2162867 (2023).
Perez-Lopez, F. R. et al. Obstetric and perinatal outcomes of pregnancies with Covid 19: a systematic review and meta-analysis. J. Matern. Fetal Neonatal Med. 35, 9742–9758 (2022).
Papapanou, M. et al. Maternal and neonatal characteristics and outcomes of Covid-19 in pregnancy: an overview of systematic reviews. Int J. Environ. Res. Public Health 18, 596 (2021).
Juan, J. et al. Effect of coronavirus disease 2019 (Covid-19) on maternal, perinatal and neonatal outcome: systematic review. Ultrasound Obstet. Gynecol. 56, 15–27 (2020).
Figueiro-Filho, E. A., Yudin, M. & Farine, D. Covid-19 during pregnancy: an overview of maternal characteristics, clinical symptoms, maternal and neonatal outcomes of 10,996 cases described in 15 countries. J. Perinat. Med. 48, 900–911 (2020).
Amaral, W. N. D. et al. Maternal coronavirus infections and neonates born to mothers with Sars-Cov-2: a systematic review. Healthc. (Basel) 8, 511 (2020).
Yee, J. et al. Clinical manifestations and perinatal outcomes of pregnant women with Covid-19: a systematic review and meta-analysis. Sci. Rep. 10, 18126 (2020).
Jafari, M. et al. Clinical characteristics and outcomes of pregnant women with Covid-19 and comparison with control patients: a systematic review and meta-analysis. Rev. Med. Virol. 31, 1–16 (2021).
Wei, S. Q., Bilodeau-Bertrand, M., Liu, S. & Auger, N. The impact of Covid-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ 193, E540–E548 (2021).
Pathirathna, M. L., Samarasekara, B. P. P., Dasanayake, T. S., Saravanakumar, P. & Weerasekara, I. Adverse perinatal outcomes in Covid-19 infected pregnant women: a systematic review and meta-analysis. Healthcare 10, 203 (2022).
Boettcher, L. B. & Metz, T. D. Maternal and neonatal outcomes following Sars-Cov-2 infection. Semin. Fetal Neonatal Med. 28, 101428 (2023).
Committee on Obstetric, P. Committee opinion no. 713: antenatal corticosteroid therapy for fetal maturation. Obstet. Gynecol. 130, e102–e109 (2017).
Seaton, C. L. et al. Coronavirus disease 2019 (Covid-19) perinatal outcomes across the pandemic at an academic medical center in New York City. Obstet. Gynecol. 141, 144–151 (2023).
Hui, L. et al. Reductions in stillbirths and preterm birth in Covid-19-vaccinated women: a multicenter cohort study of vaccination uptake and perinatal outcomes. Am. J. Obstet. Gynecol. 228, 585.e1–585.e16 (2023).
Shende, P. et al. Persistence of Sars-Cov-2 in the first trimester placenta leading to transplacental transmission and fetal demise from an asymptomatic mother. Hum. Reprod. 36, 899–906 (2021).
Stoecklein, S. et al. Effects of Sars-Cov-2 on Prenatal lung growth assessed by fetal MRI. Lancet Respir. Med. 10, e36–e37 (2022).
Kienast, P. et al. Sars-Cov-2 variant-related abnormalities detected by prenatal MRI: a prospective case-control study. Lancet Reg. Health Eur. 26, 100587 (2023).
Gubbari, C., Govindarajan, V., Reddy, C., Raman, P. & Supriya, M. Newborn with nonimmune hydrops secondary to fetal Covid-19 myocarditis. Indian J. Pediatr. 89, 99 (2022).
Sileo, F. G. et al. Pregnant woman infected by coronavirus disease (Covid-19) and calcifications of the fetal bowel and gallbladder. Minerva Obstet. Gynecol. 73, 121–124 (2021).
Chimenea, A., Garcia-Diaz, L., Calderon, A. M. & Antinolo, G. Prenatal diagnosis of Vacterl association after early-first trimester Sars-Cov-2 infection. Congenit. Anom. 63, 44–46 (2023).
Brogna, C. et al. Perinatal cerebral ischemic lesion and Sars-Cov-2 infection during pregnancy: a case report and a review of the literature. J. Clin. Med. 11, 6827 (2022).
Wang, G., Stapley, E., Peterson, S., Parrott, J. & Clark-Ganheart, C. Persistent Fetal Svt in a Covid-19 Positive Pregnancy. Case Rep. Obstet. Gynecol. 2022, 9933520 (2022).
Ozdil, M., Cetin, I. & D, A. Neonatal Case of Cerebral Venous Sinus Thrombosis with Intrauterine Onset after Covid-19 Infection During Pregnancy: Cause or Coincidence? J. Stroke Cerebrovasc. Dis. 32, 106922 (2023).
Nita, R. A. & Matulatan, F. Necrotizing enterocolitis in preterm newborn with a history of maternal Covid-19: a case report. Radio. Case Rep. 17, 2630–2634 (2022).
Oswal, J., Sarangi, B. & Badarayan, K. Infantile immune thrombocytopenic purpura secondary to perinatal transfer of Sars-Cov-2 antibody. Indian Pediatr. 59, 169–170 (2022).
Hernandez-Diaz, S., Smith, L. H., Wyszynski, D. F. & Rasmussen, S. A. First trimester Covid-19 and the risk of major congenital malformations-international registry of coronavirus exposure in pregnancy. Birth Defects Res. 114, 906–914 (2022).
Goncu Ayhan, S. et al. Influence of Covid-19 infection on fetal thymus size after recovery. J. Perinat. Med. 50, 139–143 (2022).
Grazioli, S. et al. Immunological assessment of pediatric multisystem inflammatory syndrome related to coronavirus disease 2019. J. Pediatr. Infect. Dis. Soc. 10, 706–713 (2021).
Pawar, R. et al. Neonatal Multisystem Inflammatory Syndrome (Mis-N) associated with prenatal maternal Sars-Cov-2: a case series. Children 8, 572 (2021).
Lakshminrusimha, S., More, K., Shah, P. S., Wynn, J. L. & Sanchez, P. J. Multisystem inflammatory syndrome in neonates (Mis-N) associated with perinatal sars Cov-2 infection: does it exist? Semin. Fetal Neonatal Med. 28, 101433 (2023).
Molloy, E. J., Nakra, N., Gale, C., Dimitriades, V. R. & Lakshminrusimha, S. Multisystem Inflammatory Syndrome in Children (Mis-C) and Neonates (Mis-N) associated with Covid-19: optimizing definition and management. Pediatr. Res. 93, 1499–1508 (2023).
Ramaswamy, V. V., Abiramalatha, T., Pullattayil, S. A. & Trevisanuto, D. Multisystem inflammatory disease in neonates (Mis-N) due to maternal Covid-19. Semin. Fetal Neonatal Med. 28, 101431 (2023).
Sharma, P., Gupta, R. & Mahajan, V. Multisystem inflammatory syndrome presenting as sinus bradycardia in a neonate with prenatal exposure to Covid-19. Indian J. Pediatr. 89, 825 (2022).
Kappanayil, M. et al. Multisystem inflammatory syndrome in a neonate, temporally associated with prenatal exposure to Sars-Cov-2: a case report. Lancet Child. Adolesc. Health 5, 304–308 (2021).
Moore, E. R., Bergman, N., Anderson, G. C. & Medley, N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst. Rev. 11, CD003519 (2016).
Sariboga, Y., Sir, O., Atas, S. & Demir Gokmen, B. The relationship between Covid-19 fear and prenatal attachment of pregnant women in the pandemic. Florence Nightingale J. Nurs. 30, 232–237 (2022).
Tohme, P. et al. The psychological impact of the Covid-19 outbreak on pregnancy and mother-infant prenatal bonding. Matern. Child Health J. 26, 2221–2227 (2022).
Iyengar, U., Jaiprakash, B., Haitsuka, H. & Kim, S. One Year into the pandemic: a systematic review of perinatal mental health outcomes during Covid-19. Front. Psychiatry 12, 674194 (2021).
Duguay, G. et al. Socioemotional development in infants of pregnant women during the Covid-19 pandemic: the role of prenatal and postnatal maternal distress. Child Adolesc. Psychiatry Ment. Health 16, 28 (2022).
Khan, S. et al. The Covid-19 infection in children and its association with the immune system, prenatal stress, and neurological complications. Int J. Biol. Sci. 18, 707–716 (2022).
Nabi, G. et al. Covid-19 induced psychosocial stressors during gestation: possible maternal and neonatal consequences. Curr. Med Res Opin. 36, 1633–1634 (2020).
Zimmer, A., Youngblood, A., Adnane, A., Miller, B. J. & Goldsmith, D. R. Prenatal exposure to viral infection and neuropsychiatric disorders in offspring: a review of the literature and recommendations for the Covid-19 pandemic. Brain Behav. Immun. 91, 756–770 (2021).
Granja, M. G. et al. Sars-Cov-2 infection in pregnant women: neuroimmune-endocrine changes at the maternal-fetal interface. Neuroimmunomodulation 28, 1–21 (2021).
Lukiw, W. J., Pogue, A. & Hill, J. M. Sars-Cov-2 infectivity and neurological targets in the brain. Cell Mol. Neurobiol. 42, 217–224 (2022).
Brum, A. C. & Vain, N. E. Impact of perinatal covid on fetal and neonatal brain and neurodevelopmental outcomes. Semin. Fetal Neonatal Med. 28, 101427 (2023).
Rajagopalan, V. et al. Impact of Covid-19 related maternal stress on fetal brain development: a multimodal Mri study. J. Clin. Med. 11, 6635 (2022).
Shuffrey, L. C. et al. Association of birth during the Covid-19 pandemic with neurodevelopmental status at 6 months in infants with and without in utero exposure to maternal Sars-Cov-2 infection. JAMA Pediatr. 176, e215563 (2022).
Deoni, S. C., Beauchemin, J., Volpe, A., Da Sa, V. & Consortium, R. The Covid-19 Pandemic and Early Child Cognitive Development: A Comparison of Development in Children Born During the Pandemic and Historical References. medRxiv, 2021.08.10.21261846 (2022).
Silva, P. Y. F. et al. Risk of global developmental delay in infants born from mothers with Covid-19: a cross-sectional study. Int J. Women’s Health 15, 467–474 (2023).
Ayed, M. et al. Neurodevelopmental outcomes of infants born to mothers with Sars-Cov-2 infections during pregnancy: a national prospective study in Kuwait. BMC Pediatr. 22, 319 (2022).
Buonsenso, D. et al. Short- and mid-term multidisciplinary outcomes of newborns exposed to Sars-Cov-2 in utero or during the perinatal period: preliminary findings. Eur. J. Pediatr. 181, 1507–1520 (2022).
Edlow, A. G., Castro, V. M., Shook, L. L., Kaimal, A. J. & Perlis, R. H. Neurodevelopmental outcomes at 1 year in infants of mothers who tested positive for Sars-Cov-2 during pregnancy. JAMA Netw. Open 5, e2215787 (2022).
Liu, H. Y. et al. Transient early fine motor abnormalities in infants born to Covid-19 mothers are associated with placental hypoxia and ischemia. Front Pediatr. 9, 793561 (2021).
Aldrete-Cortez, V. et al. Infants prenatally exposed to Sars-Cov-2 show the absence of fidgety movements and are at higher risk for neurological disorders: a comparative study. PLoS One 17, e0267575 (2022).
Easterlin, M. C., Crimmins, E. M. & Finch, C. E. Will prenatal exposure to Sars-Cov-2 define a birth cohort with accelerated aging in the century ahead? J. Dev. Orig. Health Dis. 12, 683–687 (2021).
Shook, L. L., Sullivan, E. L., Lo, J. O., Perlis, R. H. & Edlow, A. G. Covid-19 in pregnancy: implications for fetal brain development. Trends Mol. Med. 28, 319–330 (2022).
Barker, D. J., Osmond, C., Kajantie, E. & Eriksson, J. G. Growth and chronic disease: findings in the helsinki birth cohort. Ann. Hum. Biol. 36, 445–458 (2009).
Peng, S. et al. Genetic regulation of the placental transcriptome underlies birth weight and risk of childhood obesity. PLoS Genet 14, e1007799 (2018).
Gayen Nee’ Betal, S. et al. Covid-19 infection during pregnancy induces differential gene expression in human cord blood cells from term neonates. Front Pediatr. 10, 834771 (2022).
Ockene, M. W. et al. Accelerated longitudinal weight gain among infants with in utero Covid-19 exposure. J. Clin. Endocrinol. Metab. 28, 2579–2588 (2023).
Author information
Authors and Affiliations
Contributions
I.H.C. contributed to conception and design, acquisition of data, and analysis and interpretation of data; drafting the article and revising it critically for important intellectual content, manuscript preparation. A.T. contributed to acquisition and interpretation of data and assisted in the manuscript preparation. F.E.C. contributed to conception and design, acquisition of data, and analysis and interpretation of data; drafting the article and revising it critically for important intellectual content and assisted in the manuscript preparation. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent to participate
Patient consent was not required for this review.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Celik, I.H., Tanacan, A. & Canpolat, F.E. Neonatal outcomes of maternal prenatal coronavirus infection. Pediatr Res 95, 445–455 (2024). https://doi.org/10.1038/s41390-023-02950-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02950-2
This article is cited by
-
Trends in prenatal and pediatric viral infections, and the impact of climate change
Pediatric Research (2024)