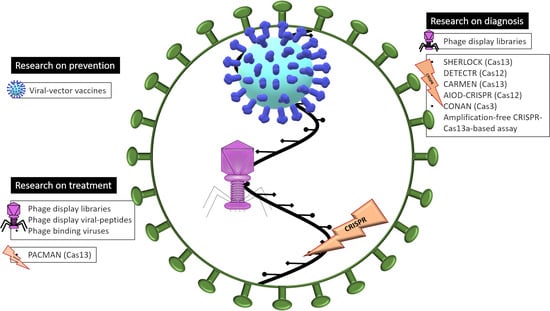
Viral Related Tools against SARS-CoV-2
Abstract
:1. Introduction
2. Human Viruses as Prevention
3. Bacteriophages
3.1. Bacteriophages as Diagnostic Tools: Phage-Display Libraries
3.2. Bacteriophages as Treatments
4. CRISPR-Cas
4.1. CRISPR-Cas as a Molecular Tool of Diagnostic of COVID-19
- (i)
- SHERLOCK: Specific High-sensitivity Enzymatic Reporter unLOCKing. This technique uses the RNAse activity of the CRISPR-Cas13a protein, which needs only a small specific RNA guide [112]. The system was adapted to a simple test against SARS-CoV-2, called STOPCovid (SHERLOCK Testing in One Pot), which counts nowadays with two versions: STOPCovid.v1 and STOPCovid.v2 [113]. Both of them use LAMP technique for RNA amplification and can detect up to 100 viral genome copies per reaction in 45–60 min. STOPCovid.v2 uses magnetic beads to simplify the RNA extraction and reduce its duration [113]. Researchers have developed a simple test format that can be performed without complex instrumentation and can detect the virus in saliva samples [114]. This method has been clinically validated by a different research group, who have decreased the limit of detection, thus increasing its sensitivity [115].
- (ii)
- DETECTR: DNA Endonuclease TargEted CRISPR Trans Reporter. This system uses the CRISPR-Cas12a protein to detect SARS-CoV-2 through its nucleoprotein and envelope genes, based on the method of RT-LAMP, which includes a simultaneous retrotranscription process. This technique allows the detection of the virus in naso- and oropharyngeal samples within 30–40 min. The limit of detection is 10 copies per microliter [116].
- (iii)
- CARMEN: Combinatorial Arrayed Reactions for Multiplexed Evaluation of Nucleic-acids. This method combines SHERLOCK with microfluidic technology, enabling the analysis of numerous types of samples from patients. The system was developed to detect 169 human-associated viruses, including SARS-CoV-2. Moreover, it can be used for viral detection in several types of samples, ranging from plasma to nasal swab samples [117].
- (iv)
- AIOD-CRISPR: All In One Dual CRISPR-Cas12a. This system uses the Cas12a protein in a fast, specific, simple method for the visual detection of SARS-CoV-2 and HIV viruses by the naked eye. This method can also be performed at a single temperature, thus avoiding the need for techniques such as LAMP. It detected 1.3 copies of a plasmid expressing the nucleocapsid protein of SARS-CoV-2, although it has not yet been tested with clinical samples [118].
- (v)
- CONAN: Cas3-Operated Nucleic Acid detectioN. This CRISPR-based tool employs mainly Cas3 endonuclease, in combination with Cas5, 6, 7, 8, and 11, which mediates targeted DNA cleavage. When combined with isothermal amplification methods, CONAN provides a rapid and sensitive method to detect SARS-CoV-2, with a reliability of 90% [119].
- (vi)
- CRISPR-COVID: A few months ago, another CRISPR-based tool suitable for the diagnostic of SARS-CoV-2 infection was developed, also based on the Cas13a endonuclease. Scientists claimed that this technique was extremely sensitive and specific, with almost a single-copy sensitivity, as they were able to identify as low as 7.5 copies of viral RNA per reaction in some cases. Furthermore, they did not detect any false positives and the time needed per reaction was only 40 min [120].
4.2. CRISPR-Cas as a Treatment
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Drosten, C.; Günther, S.; Preiser, W.; van der Werf, S.; Brodt, H.R.; Becker, S.; Rabenau, H.; Panning, M.; Kolesnikova, L.; Fouchier, R.A.; et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.N.; Sarker, M. A review of Coronavirus 2019 COVID-19 a life threating disease all over the world. World Cancer Res. J. 2020, 7, e1586. [Google Scholar] [CrossRef]
- Ahn, D.G.; Shin, H.J.; Kim, M.H.; Lee, S.; Kim, H.S.; Myoung, J.; Kim, B.T.; Kim, S.J. Current Status of Epidemiology, Diagnosis, Therapeutics, and Vaccines for Novel Coronavirus Disease 2019 (COVID-19). J. Microbiol. Biotechnol. 2020, 30, 313–324. [Google Scholar] [CrossRef]
- Wang, C.; Horby, P.W.; Hayden, F.G.; Gao, G.F. A novel coronavirus outbreak of global health concern. Lancet 2020, 395, 470–473. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Laboratory testing of 2019 novel coronavirus (2019-nCoV) in suspected human cases. In Interim Guidance; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Lisboa Bastos, M.; Tavaziva, G.; Abidi, S.K.; Campbell, J.R.; Haraoui, L.P.; Johnston, J.C.; Lan, Z.; Law, S.; MacLean, E.; Trajman, A.; et al. Diagnostic accuracy of serological tests for covid-19: Systematic review and meta-analysis. BMJ 2020, 370, m2516. [Google Scholar] [CrossRef]
- De Clercq, E. New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections. Chem. Asian J. 2019, 14, 3962–3968. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Zumla, A.; Chan, J.F.; Azhar, E.I.; Hui, D.S.; Yuen, K.Y. Coronaviruses—Drug discovery and therapeutic options. Nat. Rev. Drug Discov. 2016, 15, 327–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralinski, L.E.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, M.; De Giglio, M.A.R.; Roviello, G.N. SARS-CoV-2: Recent Reports on Antiviral Therapies Based on Lopinavir/Ritonavir, Darunavir/Umifenovir, Hydroxychloroquine, Remdesivir, Favipiravir and Other Drugs for the Treatment of the New Coronavirus. Curr. Med. Chem. 2020, 27. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.; Kalyesubula, I.; Bagenda, D.; Myers, C.; Olness, K. Effect of chloroquine on human immunodeficiency virus (HIV) vertical transmission. Afr. Health Sci. 2003, 3, 61–67. [Google Scholar] [PubMed]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freiberg, A.N.; Worthy, M.N.; Lee, B.; Holbrook, M.R. Combined chloroquine and ribavirin treatment does not prevent death in a hamster model of Nipah and Hendra virus infection. J. Gen. Virol. 2010, 91, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Dowall, S.D.; Bosworth, A.; Watson, R.; Bewley, K.; Taylor, I.; Rayner, E.; Hunter, L.; Pearson, G.; Easterbrook, L.; Pitman, J.; et al. Chloroquine inhibited Ebola virus replication in vitro but failed to protect against infection and disease in the in vivo guinea pig model. J. Gen. Virol. 2015, 96, 3484–3492. [Google Scholar] [CrossRef]
- Chu, C.M.; Cheng, V.C.; Hung, I.F.; Wong, M.M.; Chan, K.H.; Chan, K.S.; Kao, R.Y.; Poon, L.L.; Wong, C.L.; Guan, Y.; et al. Role of lopinavir/ritonavir in the treatment of SARS: Initial virological and clinical findings. Thorax 2004, 59, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Boriskin, Y.S.; Leneva, I.A.; Pecheur, E.I.; Polyak, S.J. Arbidol: A broad-spectrum antiviral compound that blocks viral fusion. Curr. Med. Chem. 2008, 15, 997–1005. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [Green Version]
- Ura, T.; Okuda, K.; Shimada, M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.H.; Keiser, M.S.; Davidson, B.L. Viral Vectors for Gene Transfer. Curr. Protoc. Mouse Biol. 2018, 8, e58. [Google Scholar] [CrossRef]
- Garretto, A.; Miller-Ensminger, T.; Wolfe, A.J.; Putonti, C. Bacteriophages of the lower urinary tract. Nat. Rev. Urol. 2019, 16, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef] [Green Version]
- Furfaro, L.L.; Payne, M.S.; Chang, B.J. Bacteriophage Therapy: Clinical Trials and Regulatory Hurdles. Front. Cell Infect. Microbiol. 2018, 8, 376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and use of Personalized Bacteriophage-Based Therapeutic Cocktails to Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [Green Version]
- Blasco, L.; Ambroa, A.; Trastoy, R.; Bleriot, I.; Moscoso, M.; Fernández-Garcia, L.; Perez-Nadales, E.; Fernández-Cuenca, F.; Torre-Cisneros, J.; Oteo-Iglesias, J.; et al. In vitro and in vivo efficacy of combinations of colistin and different endolysins against clinical strains of multi-drug resistant pathogens. Sci. Rep. 2020, 10, 7163. [Google Scholar] [CrossRef]
- Pacios, O.; Blasco, L.; Bleriot, I.; Fernandez-Garcia, L.; Gonzalez Bardanca, M.; Ambroa, A.; Lopez, M.; Bou, G.; Tomas, M. Strategies to Combat Multidrug-Resistant and Persistent Infectious Diseases. Antibiotics 2020, 9. [Google Scholar] [CrossRef] [Green Version]
- Goulart, L.R.; da, S.R.V.; Costa-Cruz, J.M. Anti-parasitic Antibodies from Phage Display. Adv. Exp. Med. Biol. 2017, 1053, 155–171. [Google Scholar] [CrossRef]
- Barbas, C.F.; Burton, D.R. Selection and evolution of high-affinity human anti-viral antibodies. Trends Biotechnol. 1996, 14, 230–234. [Google Scholar] [CrossRef]
- Mojica, F.J.; Juez, G.; Rodriguez-Valera, F. Transcription at different salinities of Haloferax mediterranei sequences adjacent to partially modified PstI sites. Mol. Microbiol. 1993, 9, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Mojica, F.J.; Diez-Villasenor, C.; Garcia-Martinez, J.; Soria, E. Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Marraffini, L.A. CRISPR-Cas systems: Prokaryotes upgrade to adaptive immunity. Mol. Cell 2014, 54, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Lander, E.S. The Heroes of CRISPR. Cell 2016, 164, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Cavazzana-Calvo, M.; Hacein-Bey, S.; de Saint Basile, G.; Gross, F.; Yvon, E.; Nusbaum, P.; Selz, F.; Hue, C.; Certain, S.; Casanova, J.L.; et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 2000, 288, 669–672. [Google Scholar] [CrossRef]
- Tebas, P.; Stein, D.; Binder-Scholl, G.; Mukherjee, R.; Brady, T.; Rebello, T.; Humeau, L.; Kalos, M.; Papasavvas, E.; Montaner, L.J.; et al. Antiviral effects of autologous CD4 T cells genetically modified with a conditionally replicating lentiviral vector expressing long antisense to HIV. Blood 2013, 121, 1524–1533. [Google Scholar] [CrossRef]
- Slobod, K.S.; Shenep, J.L.; Lujan-Zilbermann, J.; Allison, K.; Brown, B.; Scroggs, R.A.; Portner, A.; Coleclough, C.; Hurwitz, J.L. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine 2004, 22, 3182–3186. [Google Scholar] [CrossRef]
- Hansen, S.G.; Ford, J.C.; Lewis, M.S.; Ventura, A.B.; Hughes, C.M.; Coyne-Johnson, L.; Whizin, N.; Oswald, K.; Shoemaker, R.; Swanson, T.; et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 2011, 473, 523–527. [Google Scholar] [CrossRef] [Green Version]
- Rerks-Ngarm, S.; Pitisuttithum, P.; Nitayaphan, S.; Kaewkungwal, J.; Chiu, J.; Paris, R.; Premsri, N.; Namwat, C.; de Souza, M.; Adams, E.; et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 2009, 361, 2209–2220. [Google Scholar] [CrossRef]
- Coughlan, L.; Bradshaw, A.C.; Parker, A.L.; Robinson, H.; White, K.; Custers, J.; Goudsmit, J.; Van Roijen, N.; Barouch, D.H.; Nicklin, S.A.; et al. Ad5:Ad48 hexon hypervariable region substitutions lead to toxicity and increased inflammatory responses following intravenous delivery. Mol. Ther. 2012, 20, 2268–2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, V.; Petry, H.; Salmon, F. Immune Responses to AAV-Vectors, the Glybera Example from Bench to Bedside. Front. Immunol. 2014, 5, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, K.Q.; Mizukami, H.; Urabe, M.; Toda, Y.; Shinoda, K.; Yoshida, A.; Oomura, K.; Kojima, Y.; Ichino, M.; Klinman, D.; et al. Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells. J. Virol. 2006, 80, 11899–11910. [Google Scholar] [CrossRef] [Green Version]
- Perreau, M.; Pantaleo, G.; Kremer, E.J. Activation of a dendritic cell-T cell axis by Ad5 immune complexes creates an improved environment for replication of HIV in T cells. J. Exp. Med. 2008, 205, 2717–2725. [Google Scholar] [CrossRef]
- Gomez, C.E.; Najera, J.L.; Perdiguero, B.; Garcia-Arriaza, J.; Sorzano, C.O.; Jimenez, V.; Gonzalez-Sanz, R.; Jimenez, J.L.; Munoz-Fernandez, M.A.; Lopez Bernaldo de Quiros, J.C.; et al. The HIV/AIDS vaccine candidate MVA-B administered as a single immunogen in humans triggers robust, polyfunctional, and selective effector memory T cell responses to HIV-1 antigens. J. Virol. 2011, 85, 11468–11478. [Google Scholar] [CrossRef] [Green Version]
- Chiuppesi, F.; Vannucci, L.; De Luca, A.; Lai, M.; Matteoli, B.; Freer, G.; Manservigi, R.; Ceccherini-Nelli, L.; Maggi, F.; Bendinelli, M.; et al. A lentiviral vector-based, herpes simplex virus 1 (HSV-1) glycoprotein B vaccine affords cross-protection against HSV-1 and HSV-2 genital infections. J. Virol. 2012, 86, 6563–6574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, S.G.; Sacha, J.B.; Hughes, C.M.; Ford, J.C.; Burwitz, B.J.; Scholz, I.; Gilbride, R.M.; Lewis, M.S.; Gilliam, A.N.; Ventura, A.B.; et al. Cytomegalovirus vectors violate CD8 + T cell epitope recognition paradigms. Science 2013, 340, 1237874. [Google Scholar] [CrossRef] [Green Version]
- Cavenaugh, J.S.; Awi, D.; Mendy, M.; Hill, A.V.; Whittle, H.; McConkey, S.J. Partially randomized, non-blinded trial of DNA and MVA therapeutic vaccines based on hepatitis B virus surface protein for chronic HBV infection. PLoS ONE 2011, 6, e14626. [Google Scholar] [CrossRef]
- Tameris, M.D.; Hatherill, M.; Landry, B.S.; Scriba, T.J.; Snowden, M.A.; Lockhart, S.; Shea, J.E.; McClain, J.B.; Hussey, G.D.; Hanekom, W.A.; et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: A randomised, placebo-controlled phase 2b trial. Lancet 2013, 381, 1021–1028. [Google Scholar] [CrossRef] [Green Version]
- Smaill, F.; Jeyanathan, M.; Smieja, M.; Medina, M.F.; Thanthrige-Don, N.; Zganiacz, A.; Yin, C.; Heriazon, A.; Damjanovic, D.; Puri, L.; et al. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci. Transl. Med. 2013, 5, 205ra134. [Google Scholar] [CrossRef]
- Lin, J.; Calcedo, R.; Vandenberghe, L.H.; Bell, P.; Somanathan, S.; Wilson, J.M. A new genetic vaccine platform based on an adeno-associated virus isolated from a rhesus macaque. J. Virol. 2009, 83, 12738–12750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berthoud, T.K.; Hamill, M.; Lillie, P.J.; Hwenda, L.; Collins, K.A.; Ewer, K.J.; Milicic, A.; Poyntz, H.C.; Lambe, T.; Fletcher, H.A.; et al. Potent CD8 + T-cell immunogenicity in humans of a novel heterosubtypic influenza A vaccine, MVA-NP+M1. Clin. Infect. Dis. 2011, 52, 1–7. [Google Scholar] [CrossRef]
- Carter, B.J. Adeno-associated virus vectors in clinical trials. Hum. Gene Ther. 2005, 16, 541–550. [Google Scholar] [CrossRef]
- Chan, W.M.; Rahman, M.M.; McFadden, G. Oncolytic myxoma virus: The path to clinic. Vaccine 2013, 31, 4252–4258. [Google Scholar] [CrossRef] [Green Version]
- Jiang, S.; Hillyer, C.; Du, L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol. 2020, 41, 355–359. [Google Scholar] [CrossRef]
- Chiuppesi, F.; Salazar, M.D.; Contreras, H.; Nguyen, V.H.; Martinez, J.; Park, S.; Nguyen, J.; Kha, M.; Iniguez, A.; Zhou, Q.; et al. Development of a Synthetic Poxvirus-Based SARS-CoV-2 Vaccine. bioRxiv 2020. [Google Scholar] [CrossRef]
- Wold, W.S.; Toth, K. Adenovirus vectors for gene therapy, vaccination and cancer gene therapy. Curr. Gene 2013, 13, 421–433. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- US National Library of Medicine. Phase III Trial of A COVID-19 Vaccine of Adenovirus Vector in Adults 18 Years Old and above, on NIH; USA National Library of Medicine: Bethesda, MD, USA, 2020. [Google Scholar]
- Van Doremalen, N.; Lambe, T.; Spencer, A.; Belij-Rammerstorfer, S.; Purushotham, J.N.; Port, J.R.; Avanzato, V.A.; Bushmaker, T.; Flaxman, A.; Ulaszewska, M.; et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature 2020. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- US National Library of Medicine. Phase III Doubled-Blind, Placebo-Controlled Study of AZD1222 for the Prevention of COVID-19 in Adults, on NIH; USA National Library of Medicine: Bethesda, MD, USA, 2020. [Google Scholar]
- Mercado, N.B.; Zahn, R.; Wegmann, F.; Loos, C.; Chandrashekar, A.; Yu, J.; Liu, J.; Peter, L.; McMahan, K.; Tostanoski, L.H.; et al. Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature 2020. [Google Scholar] [CrossRef]
- Janssen Pharmaceutical Companies. A Study of Ad26COVS1 in Adults (COVID-19). Available online: https://clinicaltrials.gov/ct2/show/record/NCT04436276?term=NCT04436276&draw=2&rank=1 (accessed on 4 August 2020).
- Companies, J.P. A Study of Ad26.COV2.S for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adult Participants (ENSEMBLE), on NIH; US National Library of Medicine: Bethesda, MD, USA, 2020. [Google Scholar]
- Gamaleya Research Institute. An Open Study of the Safety, Tolerability and Immunogenicity of the Drug “Gam-COVID-Vac” Vaccine Against COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT04436471?term=vaccine&cond=covid-19&draw=4 (accessed on 4 August 2020).
- Gamaleya Research Institute. An Open Study of the Safety, Tolerability and Immunogenicity of “Gam-COVID-Vac Lyo” Vaccine Against COVID-19. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04437875?term=vaccine&cond=covid-19&draw=4 (accessed on 4 August 2020).
- ReiThera. European Consortium for the Fast-Track Development of a Single-Dose Adenovirus-Based COVID-19 Vaccine. Available online: https://www.reithera.com/2020/04/23/reithera-leukocare-and-univercells-announce-pan-european-consortium-for-the-fast-track-development-of-a-single-dose-adenovirus-based-covid-19-vaccine/ (accessed on 17 August 2020).
- Institute Pasteur. Clinical Trial to Evaluate the Safety and Immunogenicitiy of the COVID-19 Vaccine (COVID-19-101). Available online: https://clinicaltrials.gov/ct2/show/record/NCT04497298?term=vaccine&cond=covid-19&draw=2&rank=1 (accessed on 17 August 2020).
- Medicago Inc. Safety, Tolerability and Immunogenicinity of a Coronavirus-Like Particle COVID-19 Vaccine in Adults Aged 18–55 Years. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04450004?term=vaccine&cond=covid-19&draw=2 (accessed on 4 August 2020).
- University Xiamen. A Phase I Clinical Trial of Influenza Virus Vector COVID-19 Vaccine for Intranasal Spray; Chinese Clinical Trial Registry: Hong Kong, China, 2020. [Google Scholar]
- World Health Organization. Draft Landscape of COVID-19 Candidate Vaccines. 9 September 2020. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 17 September 2020).
- De la Cruz, V.F.; Lal, A.A.; McCutchan, T.F. Immunogenicity and epitope mapping of foreign sequences via genetically engineered filamentous phage. J. Biol. Chem. 1988, 263, 4318–4322. [Google Scholar]
- Wang, C.; Sun, X.; Suo, S.; Ren, Y.; Li, X.; Herrler, G.; Thiel, V.; Ren, X. Phages bearing affinity peptides to severe acute respiratory syndromes-associated coronavirus differentiate this virus from other viruses. J. Clin. Virol. 2013, 57, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimizadeh, W.; Rajabibazl, M. Bacteriophage vehicles for phage display: Biology, mechanism, and application. Curr. Microbiol. 2014, 69, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.D.; Hoogenboom, H.R.; Griffiths, A.D.; Winter, G. Molecular evolution of proteins on filamentous phage. Mimicking the strategy of the immune system. J. Biol. Chem. 1992, 267, 16007–16010. [Google Scholar] [PubMed]
- Christensen, D.J.; Gottlin, E.B.; Benson, R.E.; Hamilton, P.T. Phage display for target-based antibacterial drug discovery. Drug Discov. Today 2001, 6, 721–727. [Google Scholar] [CrossRef]
- Kretzschmar, T.; von Ruden, T. Antibody discovery: Phage display. Curr. Opin. Biotechnol. 2002, 13, 598–602. [Google Scholar] [CrossRef]
- Hong, S.S.; Boulanger, P. Protein ligands of the human adenovirus type 2 outer capsid identified by biopanning of a phage-displayed peptide library on separate domains of wild-type and mutant penton capsomers. EMBO J. 1995, 14, 4714–4727. [Google Scholar] [CrossRef]
- Dyson, M.R.; Murray, K. Selection of peptide inhibitors of interactions involved in complex protein assemblies: Association of the core and surface antigens of hepatitis B virus. Proc. Natl. Acad. Sci. USA 1995, 92, 2194–2198. [Google Scholar] [CrossRef] [Green Version]
- Larson, R.S.; Brown, D.C.; Ye, C.; Hjelle, B. Peptide antagonists that inhibit Sin Nombre virus and hantaan virus entry through the beta3-integrin receptor. J. Virol. 2005, 79, 7319–7326. [Google Scholar] [CrossRef] [Green Version]
- Hall, P.R.; Hjelle, B.; Njus, H.; Ye, C.; Bondu-Hawkins, V.; Brown, D.C.; Kilpatrick, K.A.; Larson, R.S. Phage display selection of cyclic peptides that inhibit Andes virus infection. J. Virol. 2009, 83, 8965–8969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, H.; Lu, H.; Qiu, H.J.; Petrenko, V.; Liu, A. Phagemid vectors for phage display: Properties, characteristics and construction. J. Mol. Biol. 2012, 417, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Marintcheva, B. Chapter 5—Phage Display. In Harnessing the Power of Viruses; Elsevier: Amsterdam, The Netherlands, 2018; Volume 2018, pp. 133–160. [Google Scholar]
- Hess, G.T.; Cragnolini, J.J.; Popp, M.W.; Allen, M.A.; Dougan, S.K.; Spooner, E.; Ploegh, H.L.; Belcher, A.M.; Guimaraes, C.P. M13 bacteriophage display framework that allows sortase-mediated modification of surface-accessible phage proteins. Bioconjug. Chem. 2012, 23, 1478–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Tu, C.; Yu, X.; Zhang, M.; Zhang, N.; Zhao, M.; Nie, W.; Ren, Z. Bacteriophage T4 nanoparticle capsid surface SOC and HOC bipartite display with enhanced classical swine fever virus immunogenicity: A powerful immunological approach. J. Virol. Methods 2007, 139, 50–60. [Google Scholar] [CrossRef]
- Krumpe, L.R.; Atkinson, A.J.; Smythers, G.W.; Kandel, A.; Schumacher, K.M.; McMahon, J.B.; Makowski, L.; Mori, T. T7 lytic phage-displayed peptide libraries exhibit less sequence bias than M13 filamentous phage-displayed peptide libraries. Proteomics 2006, 6, 4210–4222. [Google Scholar] [CrossRef]
- Cicchini, C.; Ansuini, H.; Amicone, L.; Alonzi, T.; Nicosia, A.; Cortese, R.; Tripodi, M.; Luzzago, A. Searching for DNA-protein interactions by lambda phage display. J. Mol. Biol. 2002, 322, 697–706. [Google Scholar] [CrossRef]
- Bazan, J.; Calkosinski, I.; Gamian, A. Phage display—A powerful technique for immunotherapy: 1. Introduction and potential of therapeutic applications. Hum. Vaccin. Immunother. 2012, 8, 1817–1828. [Google Scholar] [CrossRef] [Green Version]
- Chakravarthy, B.; Ménard, M.; Brown, L.; Atkinson, T.; Whitfield, J. Identification of protein kinase C inhibitory activity associated with a polypeptide isolated from a phage display system with homology to PCM-1, the pericentriolar material-1 protein. Biochem. Biophys. Res. Commun. 2012, 424, 147–151. [Google Scholar] [CrossRef]
- Liu, Z.X.; Yi, G.H.; Qi, Y.P.; Liu, Y.L.; Yan, J.P.; Qian, J.; Du, E.Q.; Ling, W.F. Identification of single-chain antibody fragments specific against SARS-associated coronavirus from phage-displayed antibody library. Biochem. Biophys. Res. Commun 2005, 329, 437–444. [Google Scholar] [CrossRef]
- Lim, C.C.; Woo, P.C.Y.; Lim, T.S. Development of a Phage Display Panning Strategy Utilizing Crude Antigens: Isolation of MERS-CoV Nucleoprotein human antibodies. Sci. Rep. 2019, 9, 6088. [Google Scholar] [CrossRef] [Green Version]
- Duan, J.; Yan, X.; Guo, X.; Cao, W.; Han, W.; Qi, C.; Feng, J.; Yang, D.; Gao, G.; Jin, G. A human SARS-CoV neutralizing antibody against epitope on S2 protein. Biochem. Biophys. Res. Commun. 2005, 333, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Yang, B.A.; Hu, Y.; Zhao, H.; Xiong, W.; Yang, Y.; Si, B.; Zhu, Q. Human neutralizing Fab molecules against severe acute respiratory syndrome coronavirus generated by phage display. Clin. Vaccine Immunol. 2006, 13, 953–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Begum, N.; Horiuchi, S.; Tanaka, Y.; Yamamoto, N.; Ichiyama, K.; Yamamoto, N. New approach for generation of neutralizing antibody against human T-cell leukaemia virus type-I (HTLV-I) using phage clones. Vaccine 2002, 20, 1281–1289. [Google Scholar] [CrossRef]
- Wu, Y.; Li, C.; Xia, S.; Tian, X.; Kong, Y.; Wang, Z.; Gu, C.; Zhang, R.; Tu, C.; Xie, Y.; et al. Identification of Human Single-Domain Antibodies against SARS-CoV-2. Cell Host Microbe 2020, 27, 891–898.e895. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, L.; Lin, J.; Li, X.; Liu, B.; Kong, Y.; Zeng, S.; Du, J.; Xiao, H.; Zhang, T.; et al. Blocking antibodies against SARS-CoV-2 RBD isolated from a phage display antibody library using a competitive biopanning strategy. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Liu, B.; Yin, J.; Zhang, H.; Li, G. Phage displayed peptides recognizing porcine aminopeptidase N inhibit transmissible gastroenteritis coronavirus infection in vitro. Virology 2011, 410, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauster, D.; Klenk, S.; Ludwig, K.; Nojoumi, S.; Behren, S.; Adam, L.; Stadtmüller, M.; Saenger, S.; Zimmler, S.; Hönzke, K.; et al. Phage capsid nanoparticles with defined ligand arrangement block influenza virus entry. Nat. Nanotechnol. 2020, 15, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Marraffini, L.A.; Sontheimer, E.J. CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA. Science 2008, 322, 1843–1845. [Google Scholar] [CrossRef] [Green Version]
- Deveau, H.; Barrangou, R.; Garneau, J.E.; Labonte, J.; Fremaux, C.; Boyaval, P.; Romero, D.A.; Horvath, P.; Moineau, S. Phage response to CRISPR-encoded resistance in Streptococcus thermophilus. J. Bacteriol. 2008, 190, 1390–1400. [Google Scholar] [CrossRef] [Green Version]
- Horvath, P.; Romero, D.A.; Coute-Monvoisin, A.C.; Richards, M.; Deveau, H.; Moineau, S.; Boyaval, P.; Fremaux, C.; Barrangou, R. Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus. J. Bacteriol. 2008, 190, 1401–1412. [Google Scholar] [CrossRef] [Green Version]
- Pardee, K.; Green, A.A.; Takahashi, M.K.; Braff, D.; Lambert, G.; Lee, J.W.; Ferrante, T.; Ma, D.; Donghia, N.; Fan, M.; et al. Rapid, Low-Cost Detection of Zika Virus Using Programmable Biomolecular Components. Cell 2016, 165, 1255–1266. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef] [Green Version]
- He, Q.; Yu, D.; Bao, M.; Korensky, G.; Chen, J.; Shin, M.; Kim, J.; Park, M.; Qin, P.; Du, K. High-throughput and all-solution phase African Swine Fever Virus (ASFV) detection using CRISPR-Cas12a and fluorescence based point-of-care system. Biosens. Bioelectron. 2020, 154, 112068. [Google Scholar] [CrossRef]
- Guk, K.; Keem, J.O.; Hwang, S.G.; Kim, H.; Kang, T.; Lim, E.K.; Jung, J. A facile, rapid and sensitive detection of MRSA using a CRISPR-mediated DNA FISH method, antibody-like dCas9/sgRNA complex. Biosens. Bioelectron. 2017, 95, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.; Barrangou, R. COVID-19 and the CRISPR Community Response. Cris. J. 2020, 3, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shang, X.; Huang, X. Next-generation pathogen diagnosis with CRISPR/Cas-based detection methods. Emerg. Microbes Infect. 2020, 9, 1682–1691. [Google Scholar] [CrossRef]
- Li, J.J.; Xiong, C.; Liu, Y.; Liang, J.S.; Zhou, X.W. Loop-Mediated Isothermal Amplification (LAMP): Emergence as an Alternative Technology for Herbal Medicine Identification. Front. Plant Sci. 2016, 7, 1956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellner, M.J.; Koob, J.G.; Gootenberg, J.S.; Abudayyeh, O.O.; Zhang, F. SHERLOCK: Nucleic acid detection with CRISPR nucleases. Nat. Protoc. 2019, 14, 2986–3012. [Google Scholar] [CrossRef]
- Joung, J.; Ladha, A.; Saito, M.; Kim, N.G.; Woolley, A.E.; Segel, M.; Barretto, R.P.J.; Ranu, A.; Macrae, R.K.; Faure, G.; et al. Detection of SARS-CoV-2 with SHERLOCK One-Pot Testing. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Joung, J.; Ladha, A.; Saito, M.; Segel, M.; Bruneau, R.; Huang, M.-l.W.; Kim, N.-G.; Yu, X.; Li, J.; Walker, B.D.; et al. Point-of-care testing for COVID-19 using SHERLOCK diagnostics. MedRxiv 2020. [Google Scholar] [CrossRef]
- Patchsung, M.; Jantarug, K.; Pattama, A.; Aphicho, K.; Suraritdechachai, S.; Meesawat, P.; Sappakhaw, K.; Leelahakorn, N.; Ruenkam, T.; Wongsatit, T.; et al. Clinical validation of a Cas13-based assay for the detection of SARS-CoV-2 RNA. Nat. Biomed. Eng. 2020. [Google Scholar] [CrossRef] [PubMed]
- Broughton, J.P.; Deng, X.; Yu, G.; Fasching, C.L.; Servellita, V.; Singh, J.; Miao, X.; Streithorst, J.A.; Granados, A.; Sotomayor-Gonzalez, A.; et al. CRISPR-Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870–874. [Google Scholar] [CrossRef] [Green Version]
- Ackerman, C.M.; Myhrvold, C.; Thakku, S.G.; Freije, C.A.; Metsky, H.C.; Yang, D.K.; Ye, S.H.; Boehm, C.K.; Kosoko-Thoroddsen, T.-S.F.; Kehe, J.; et al. Massively multiplexed nucleic acid detection using Cas13. Nature 2020, 582, 277–282. [Google Scholar] [CrossRef]
- Ding, X.; Yin, K.; Li, Z.; Liu, C. All-in-One Dual CRISPR-Cas12a (AIOD-CRISPR) Assay: A Case for Rapid, Ultrasensitive and Visual Detection of Novel Coronavirus SARS-CoV-2 and HIV virus. bioRxiv 2020. [Google Scholar] [CrossRef]
- Yoshimi, K.; Takeshita, K.; Yamayoshi, S.; Shibumura, S.; Yamauchi, Y.; Yamamoto, M.; Yotsuyanagi, H.; Kawaoka, Y.; Mashimo, T. Rapid and accurate detection of novel coronavirus SARS-CoV-2 using CRISPR-Cas3. MedRxiv 2020. [Google Scholar] [CrossRef]
- Hou, T.; Zeng, W.; Yang, M.; Chen, W.; Ren, L.; Ai, J.; Wu, J.; Liao, Y.; Gou, X.; Li, Y.; et al. Development and evaluation of a rapid CRISPR-based diagnostic for COVID-19. PLoS Pathog. 2020, 16, e1008705. [Google Scholar] [CrossRef]
- Abbott, T.R.; Dhamdhere, G.; Liu, Y.; Lin, X.; Goudy, L.; Zeng, L.; Chemparathy, A.; Chmura, S.; Heaton, N.S.; Debs, R.; et al. Development of CRISPR as an Antiviral Strategy to Combat SARS-CoV-2 and Influenza. Cell 2020, 181, 865–876. [Google Scholar] [CrossRef]
- Fozouni, P.; Son, S.; de León Derby, M.D.; Knott, G.J.; Gray, C.N.; D’Ambrosio, M.V.; Zhao, C.; Switz, N.A.; Kumar, G.R.; Stephens, S.I.; et al. Direct detection of SARS-CoV-2 using CRISPR-Cas13a and a mobile phone. MedRxiv 2020. [Google Scholar] [CrossRef]
- Nguyen, T.M.; Zhang, Y.; Pandolfi, P.P. Virus against virus: A potential treatment for 2019-nCov (SARS-CoV-2) and other RNA viruses. Cell Res. 2020, 30, 189–190. [Google Scholar] [CrossRef] [Green Version]
- USA Food and Drugs Agency. First FDA-Approved Vaccine for the Prevention of Ebola Virus Disease, Marking a Critical Milestone in Public Health Preparedness and Response. Available online: https://www.fda.gov/news-events/press-announcements/first-fda-approved-vaccine-prevention-ebola-virus-disease-marking-critical-milestone-public-health (accessed on 13 October 2020).
- Coughlan, L. Factors Which Contribute to the Immunogenicity of Non-replicating Adenoviral Vectored Vaccines. Front. Immunol. 2020, 11, 909. [Google Scholar] [CrossRef] [PubMed]
- Cooney, E.L.; Collier, A.C.; Greenberg, P.D.; Coombs, R.W.; Zarling, J.; Arditti, D.E.; Hoffman, M.C.; Hu, S.L.; Corey, L. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet 1991, 337, 567–572. [Google Scholar] [CrossRef]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing Covid-19 Vaccines at Pandemic Speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.H.; Chen, Q.; Gu, H.J.; Yang, G.; Wang, Y.X.; Huang, X.Y.; Liu, S.S.; Zhang, N.N.; Li, X.F.; Xiong, R.; et al. A Mouse Model of SARS-CoV-2 Infection and Pathogenesis. Cell Host Microbe 2020, 28, 124–133.e124. [Google Scholar] [CrossRef]
- Sun, J.; Zhuang, Z.; Zheng, J.; Li, K.; Wong, R.L.; Liu, D.; Huang, J.; He, J.; Zhu, A.; Zhao, J.; et al. Generation of a Broadly Useful Model for COVID-19 Pathogenesis, Vaccination, and Treatment. Cell 2020. [Google Scholar] [CrossRef]
- Latrofa, F.; Pichurin, P.; Guo, J.; Rapoport, B.; McLachlan, S.M. Thyroglobulin-thyroperoxidase autoantibodies are polyreactive, not bispecific: Analysis using human monoclonal autoantibodies. J. Clin. Endocrinol. Metab. 2003, 88, 371–378. [Google Scholar] [CrossRef] [Green Version]
- Payne, A.S.; Ishii, K.; Kacir, S.; Lin, C.; Li, H.; Hanakawa, Y.; Tsunoda, K.; Amagai, M.; Stanley, J.R.; Siegel, D.L. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J. Clin. Investig. 2005, 115, 888–899. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, N.; Im, S.H.; Balass, M.; Fuchs, S.; Katchalski-Katzir, E. Prevention of passively transferred experimental autoimmune myasthenia gravis by a phage library-derived cyclic peptide. Proc. Natl. Acad. Sci. USA 2000, 97, 761–766. [Google Scholar] [CrossRef] [Green Version]
- Houimel, M.; Corthesy-Theulaz, I.; Fisch, I.; Wong, C.; Corthesy, B.; Mach, J.; Finnern, R. Selection of human single chain Fv antibody fragments binding and inhibiting Helicobacter pylori urease. Tumour. Biol. 2001, 22, 36–44. [Google Scholar] [CrossRef]
- Molina-Lopez, J.; Sanschagrin, F.; Levesque, R.C. A peptide inhibitor of MurA UDP-N-acetylglucosamine enolpyruvyl transferase: The first committed step in peptidoglycan biosynthesis. Peptides 2006, 27, 3115–3121. [Google Scholar] [CrossRef]
- Yacoby, I.; Shamis, M.; Bar, H.; Shabat, D.; Benhar, I. Targeting antibacterial agents by using drug-carrying filamentous bacteriophages. Antimicrob. Agents Chemother. 2006, 50, 2087–2097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, E.A.; Chavez-Fumagalli, M.A.; Costa, L.E.; Tavares, C.A.; Soto, M.; Goulart, L.R. Theranostic applications of phage display to control leishmaniasis: Selection of biomarkers for serodiagnostics, vaccination, and immunotherapy. Rev. Soc. Bras. Med. Trop. 2015, 48, 370–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsson, F.; Trilling, M.; Perez, F.; Ohlin, M. A dimerized single-chain variable fragment system for the assessment of neutralizing activity of phage display-selected antibody fragments specific for cytomegalovirus. J. Immunol. Methods 2012, 376, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Yin, J.; Chen, W.Q.; Jiang, M.; Yang, G.; Yang, Z.H. Generation and characterization of human monoclonal antibodies to G5, a linear neutralization epitope on glycoprotein of rabies virus, by phage display technology. Microbiol. Immunol. 2008, 52, 89–93. [Google Scholar] [CrossRef]
- Rahbarnia, L.; Farajnia, S.; Babaei, H.; Majidi, J.; Veisi, K.; Ahmadzadeh, V.; Akbari, B. Evolution of phage display technology: From discovery to application. J Drug Target 2017, 25, 216–224. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Wang, J.; Liu, G. CRISPR/Cas Systems towards Next-Generation Biosensing. Trends Biotechnol. 2019, 37, 730–743. [Google Scholar] [CrossRef]
- Citorik, R.J.; Mimee, M.; Lu, T.K. Sequence-specific antimicrobials using efficiently delivered RNA-guided nucleases. Nat. Biotechnol. 2014, 32, 1141–1145. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.L.; Ruan, M.Z.C.; Mahajan, V.B.; Tsang, S.H. Viral Delivery Systems for CRISPR. Viruses 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Jiang, W.; Marraffini, L.A. CRISPR-Cas: New Tools for Genetic Manipulations from Bacterial Immunity Systems. Annu. Rev. Microbiol. 2015, 69, 209–228. [Google Scholar] [CrossRef]
- Jackson, R.N.; Golden, S.M.; van Erp, P.B.; Carter, J.; Westra, E.R.; Brouns, S.J.; van der Oost, J.; Terwilliger, T.C.; Read, R.J.; Wiedenheft, B. Structural biology. Crystal structure of the CRISPR RNA-guided surveillance complex from Escherichia coli. Science 2014, 345, 1473–1479. [Google Scholar] [CrossRef] [Green Version]
- Gasiunas, G.; Barrangou, R.; Horvath, P.; Siksnys, V. Cas9-crRNA ribonucleoprotein complex mediates specific DNA cleavage for adaptive immunity in bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, E2579–E2586. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Developer Institution | Country/s | Type of Viral-Vector | Current State |
|---|---|---|---|
| University of Oxford/ AstraZeneca | United Kingdom | ChAdOx1-S | Clinical trial Phase 3 |
| Beijing Institute of Biotechnology/ CanSino Biological Inc. | China | Ad5 | Clinical trial Phase 2 |
| Janssen Pharmaceutical Companies | Belgium | Ad26 | Clinical trial Phase ½ |
| Gamaleya Research Institute | Russia | Adenovirus | Clinical trial Phase 1 |
| ReiThera/LEUKOCARE/Uncercells | Italy/Germany/Belgium | Adenovirus | Clinical trial Phase 1 |
| Institute Pasteur/Themis/Univ. of Pittsburgh CVR/Merck Sharp & Dohme | France/United States | Measles | Clinical trial Phase 1 |
| Medicago Inc. | Canada | Plant-derivated VLP | Clinical trial Phase 1 |
| ID Pharma | Japan | Sendai virus | Pre-clinical |
| Ankara University | Turkey | Adenovirus | Pre-clinical |
| Massachusetts General Hospital/Massachusetts Eye and Ear/AveXis | United States | Adenovirus | Pre-clinical |
| GeoVax/BravoVax | United States/China | MVA | Pre-clinical |
| German center for infection Research/IDT Biologike GmbH | Germany | MVA | Pre-clinical |
| IDIBAPS-Hospital clinic | Spain | MVA | Pre-clinical |
| Altimmune | United States | Adenovirus | Pre-clinical |
| Erciyes University | Turkey | Ad5 | Pre-clinical |
| ImmunityBio Inc/NantKwest Inc. | United States | Ad5 | Pre-clinical |
| Greffex | United States | Ad5 | Pre-clinical |
| Stabilitech Biopharma Ltd. | United Kingdom | Ad5 | Pre-clinical |
| Valo Therapeutics Ltd. | United Kingdom | Adenovirus | Pre-clinical |
| Vaxart | United States | Ad5 | Pre-clinical |
| National Biotechnology Center (CNB-CSIC) | Spain | MVA | Pre-clinical |
| University of Georgia/ University of Iowa | United States | Parainfluenza virus | Pre-clinical |
| Bharat Biotech/Thomas Jefferson University | India/United States | Rabies virus | Pre-clinical |
| National Research Centre | Egypt | Influenza A | Pre-clinical |
| National Center for Genetic Engineering and Biotechnology (BIOTEC)/ GPO | Thailand | Flu virus | Pre-clinical |
| KU Leuven | Belgium | YF17D | Pre-clinical |
| Cadila Healthcare Limited | India | Measles | Pre-clinical |
| FBRI SRC VB Vector/ Rospotrebnadzor | Russia | Measles | Pre-clinical |
| German center for infection Research/ CanVirex AG | Germany | Measles | Pre-clinical |
| Tonix Pharma/ Southern Research | United States | Horsepox | Pre-clinical |
| BiOCAD/ IEM | Russia | Influenza | Pre-clinical |
| FBRI SRC VB Vector/ Rospotrebnadzor | Russia | Influenza A | Pre-clinical |
| Fundação Oswaldo Cruz/ Instituto Buntantan | Brazil | Influenza | Pre-clinical |
| University of Hong Kong | China | Influenza | Pre-clinical |
| IAVI/ Merk | Italy/United States | VSV | Pre-clinical |
| University of Manitoba | Canada | VSV | Pre-clinical |
| University of Western Ontario | United States | VSV | Pre-clinical |
| Aurobindo Pharma | India | VSV | Pre-clinical |
| FBRI SRC VB Vector/ Rospotrebnadzor | Russia | VSV | Pre-clinical |
| Israel Institute for Biological Research/ Weizman Institute of Science | Israel | VSV | Pre-clinical |
| UW-Madison/FluGen/Bharat Biotech | United States | Influenza | Pre-clinical |
| Intravacc/Wageningen Bioveterinary Research/Utrecht University | The Netherlands | Newcastle disease virus | Pre-clinical |
| The Lancaster University | United Kingdom | Avian paramyxovirus | Pre-clinical |
| University of Manitoba | Canada | VLP | Pre-clinical |
| Bezmialem Vakif University | Turkey | VLP | Pre-clinical |
| Middle East Technical University | Turkey | VLP | Pre-clinical |
| VBI Vaccines Inc. | United States | VLP | Pre-clinical |
| IrsiCaixa AIDS Research/IRTA-CReSA/Barcelona Supercomputing Centre/Grifols | Spain | VLP | Pre-clinical |
| Mahidol University/The Government Pharmaceutical Organization (GPO)/Siriraj Hospital | Thailand | VLP | Pre-clinical |
| Navarrabiomed, Oncoinmunology group | Spain | VLP | Pre-clinical |
| Saiba GmbH | Switzerland | VLP | Pre-clinical |
| Imophoron Ltd. and Bristol University’s Max Planck Centre | United Kingdom | VLP | Pre-clinical |
| Doherty Institute | Australia | VLP | Pre-clinical |
| OSIVAX | France | VLP | Pre-clinical |
| ARTES Biotechnology | Germany | VLP | Pre-clinical |
| University of Sao Paulo | Brazil | VLP | Pre-clinical |
| COVID-19 RT-PCR | STOP-Covid a (SHERLOCK) | DETECTR | CARMEN | AIOD-CRISPR | CONAN | CRISPR-COVID | |
| Gene Target | Spike protein RdRp Nucleocapsid | Spike ORF1ab Nucleocapsid | Envelop Nucleocapsid | ORF1ab | Nucleocapsid | Nucleocapsid | ORF1ab Nucleocapsid |
| Sample type | RNA | RNA | DNA | RNA | DNA | DNA | RNA |
| Assay reaction time | 120 min | 60 min | 30–40 min | ~30 min | 40 min | 30–40 min | 40 min |
| Nº of samples/ reaction | 1 | 1 | 1 | 1000 | 1 | 1 | 1 |
| Results | Quantitative | Semi-quantitative | Qualitative | Quantitative | Quantitative | Quantitative | Qualitative |
| Detection limit | >10 viral copies | 42 viral copies | 10 viral copies | 10 viral copies | 1.3 copies of SARS-CoV-2 Nucleocapsid gene plasmids | 100 viral copies | 7.5 viral copies |
| FDA Approval | Yes | Yes | In process | In process | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Garcia, L.; Pacios, O.; González-Bardanca, M.; Blasco, L.; Bleriot, I.; Ambroa, A.; López, M.; Bou, G.; Tomás, M. Viral Related Tools against SARS-CoV-2. Viruses 2020, 12, 1172. https://doi.org/10.3390/v12101172
Fernandez-Garcia L, Pacios O, González-Bardanca M, Blasco L, Bleriot I, Ambroa A, López M, Bou G, Tomás M. Viral Related Tools against SARS-CoV-2. Viruses. 2020; 12(10):1172. https://doi.org/10.3390/v12101172
Chicago/Turabian StyleFernandez-Garcia, Laura, Olga Pacios, Mónica González-Bardanca, Lucia Blasco, Inés Bleriot, Antón Ambroa, María López, German Bou, and Maria Tomás. 2020. "Viral Related Tools against SARS-CoV-2" Viruses 12, no. 10: 1172. https://doi.org/10.3390/v12101172