Abstract
This study aimed to assess pulmonary changes at 6-month follow-up CT and predictors of pulmonary residual abnormalities and fibrotic-like changes in COVID-19 pneumonia patients in China following relaxation of COVID restrictions in 2022. A total of 271 hospitalized patients with COVID-19 pneumonia admitted between November 29, 2022 and February 10, 2023 were prospectively evaluated at 6 months. CT characteristics and Chest CT scores of pulmonary abnormalities were compared between the initial and the 6-month CT. The association of demographic and clinical factors with CT residual abnormalities or fibrotic-like changes were assessed using logistic regression. Follow-up CT scans were obtained at a median of 177 days (IQR, 170–185 days) after hospital admission. Pulmonary residual abnormalities and fibrotic-like changes were found in 98 (36.2%) and 39 (14.4%) participants. In multivariable analysis of pulmonary residual abnormalities and fibrotic-like changes, the top three predictive factors were invasive ventilation (OR 13.6; 95% CI 1.9, 45; P < .001), age > 60 years (OR 9.1; 95% CI 2.3, 39; P = .01), paxlovid (OR 0.11; 95% CI 0.04, 0.48; P = .01) and invasive ventilation (OR 10.3; 95% CI 2.9, 33; P = .002), paxlovid (OR 0.1; 95% CI 0.03, 0.48; P = .01), smoker (OR 9.9; 95% CI 2.4, 31; P = .01), respectively. The 6-month follow-up CT of recent COVID-19 pneumonia cases in China showed a considerable proportion of the patients with pulmonary residual abnormalities and fibrotic-like changes. Antivirals against SARS-CoV-2 like paxlovid may be beneficial for long-term regression of COVID-19 pneumonia.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2), has become a global pandemic for more than three years. COVID-19 has been proven to cause multi-organ damage, and pneumonia is the most common manifestation1,2. Chest CT plays an important role in the diagnosis, follow-up and presumed prognosis of patients with COVID-193,4. Several studies have demonstrated permanent radiographic changes and possibility of pulmonary fibrosis during the follow-up CTs of COVID-19 patients5,6,7.
Since the beginning of the pandemic, SARS-CoV-2 has been evolving rapidly through genetic mutations during virus replication8,9. Although much evidence suggests that the pathogenicity of the virus is gradually diminishing as it evolves, the trend is not definitive10,11. Therefore, the clinical characteristics and long-term follow-up of COVID-19 still deserve continued attention.
China has experienced an outbreak of infections since the gradual deregulation of COVID-19 in the year-end of 2022. Beijing was one of the first and most severe outbreak areas, and the predominant SARS-CoV-2 variants at that time was Omicron BF.7 and BF.5.2, which had many new epidemiological and clinical characteristics12,13. This study aimed to assess pulmonary changes at 6-month follow-up CT and predictors of pulmonary residual abnormalities and fibrotic-like changes in recent COVID-19 pneumonia patients.
Materials and methods
Participants
This prospective study was approved by the Institutional Review Board of the 305 Hospital of PLA and the 7th Medical Center of Chinese PLA General Hospital. Informed consent was provided by all participants and the study was conducted in accordance with the Declaration of Helsinki.
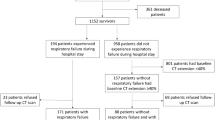
We prospectively enrolled 271 patients with COVID-19 pneumonia who had been admitted to either the 305 Hospital of PLA or the 7th Medical Center of Chinese PLA General Hospital between November 29, 2022 and February 10, 2023, when COVID-19 infections increased following gradual deregulation in China. All participants were diagnosis of COVID-19 pneumonia confirmed by means of a SARS-CoV-2 positive polymerase chain reaction test via nasopharyngeal swabs and underwent an initial chest CT to confirm the pneumonia. All positive specimens sent to the Centers for Disease Control and Prevention (CDC) were confirmed to be Omicron BF.7 and BF.5.2. The exclusion criteria included: age less than 18 years, reinfection with COVID-19 pneumonia or other lung disease during the 6-month follow-up period, refusal to be followed up or (and) inability to be contacted, and poor CT images quality (Fig. 1).
CT protocol
Non-contrast chest scans were obtained with a 64-section multidetector CT scanner (LightSpeed VCT; GE Medical Systems) or a 128-section multidetector CT scanner (Brilliance iCT; Philips Healthcare), with participants in the supine position during a breath hold following full inspiration. The scanning parameters were 120 kV and adaptive tube current, with the smallest field of view possible according to the body habitus. Axial reconstructions were performed with a section thickness of 1 or 1.25 mm using a bone filter. All 271 patients underwent initial CT scans and 6-month follow-up CT scans using the same parameters.
Image interpretation
All CT images were reviewed in random order by three radiologists (Y.Z, L.L, W.L, with 18, 17 and 10 years of experience in thoracic radiology, respectively), who were blinded to the baseline and clinical information of the participants. The readers independently assessed the CT features using axial images. Multiplane reconstruction is used to resolve any interpretive doubts. Images were interpreted at a window of 1000 to 2000 Hounsfield units and a level of − 700 to − 500 Hounsfield units, respectively, to assess the lung parenchyma.
CT features were described according to the Fleischner Society glossary14 as follows: ground-glass opacities (GGO), consolidation, reticulation, linear atelectasis, traction bronchiectasis, parenchymal bands, honeycombing, acute respiratory distress syndrome (ARDS) pattern, crazy paving pattern, organizing pneumonia and pleural effusion. The CT evidence of pulmonary fibrotic-like changes was defined as presence of linear atelectasis, traction bronchiectasis, parenchymal bands and honeycombing7,14,15.
The chest CT score is calculated per each of the five lung lobes based on the extent of parenchymal involvement16, as follows: (0) no involvement; (1) < 5% involvement; (2) 5–25% involvement; (3) 26–50% involvement; (4) 51–75% involvement; and (5) > 75% involvement. The resulting total CT score is the sum of each individual lobar score and ranges from 0 to 25.
Statistical analysis
The statistical analyses were performed using the software SPSS 25.0 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as medians with interquartile ranges (IQRs) or means ± standard deviations (SDs). Categorical variables are reported as numbers and percentages. Continuous variables with normally and nonnormally distributed data were assessed using the two-sample t test or Mann–Whitney U test, respectively. Univariable and multivariable logistic regression analyses were performed to identify the predictive factors of abnormalities or fibrotic-like changes. Statistically significant difference was considered at P < 0.05 (two tailed). Bonferroni correction was used as appropriate.
Result
Participant characteristics
A total of 271 participants (mean ± SD, 61 years ± 12) were assessed, and 113 participants were women (41.7%). The baseline and clinical characteristics are summarized in Table 1. Of the 271 participants, the median body mass index was 21.8 kg/m2 (IQR, 17.1–29.1), and 80 (29.5%) were smokers. 148 participants (54.6%) had different types of comorbidities and common comorbidities included hypertension (82 participants, 30.3%), type II diabetes mellitus (80 participants, 29.5%), ischemic heart disease (61 participants, 22.5%), chronic obstructive pulmonary disease (18 participants, 6.6%) and previous venous thromboembolism (10 participants, 3.7%). The median hospital stay was 12 days (IQR, 4–20 days), with 68 participants (25.1%) requiring the highest level of ventilatory support in the form of invasive ventilation or noninvasive positive pressure ventilation. Participants are treated with medications mainly including paxlovid (183 participants, 67.5%), azvudine (60 participants, 22.1%) and glucocorticoid (69 participants, 25.5%).
Compared of baseline and clinical characteristics, age (mean, 58 years ± 11 vs 65 years ± 12, P < 0.001), smoker (42 participants [24.3%] vs 38 participants [38.8%], P = 0.04), heart rate (mean, 83 times per minute ± 14 vs 92 times per minute ± 16, P = 0.02), respiratory rate (mean, 20 times per minute ± 7 vs 24 times per minute ± 9, P = 0.03), oxygen saturation on room air (SaO2, 96%, IQR, 88–99% vs 92%, IQR, 80–98%, P = 0.001), chronic obstructive pulmonary disease (COPD, 10 participants [5.8%] vs 8 participants [8.1%], P = 0.02), length of hospital stay (11 days, IQR, 4–14 days vs 16 days, IQR, 10–27 days, P < 0.001), invasive ventilation (2 participants [1.6%] vs 15 participants [15.3%], P < 0.001) and using paxlovid (147 participants [85.0%] vs 36 participants [36.7%], P < 0.001) demonstrated a statistically significant difference between participants with normal and abnormal chest CT at 6-month follow-up.
Comparison of CT findings
All participants underwent a 6-month follow-up chest CT at a median of 177 days (IQR, 155–203 days) after hospital admission and pulmonary residual abnormalities were found in 98 participants (36.2%). Compared to the initial CT (Table 2), participants with GGO decreased from 270 (99.6%) to 66 (24.4%) and consolidation decreased from 111 (41.0%) to 20 (7.4%) (Fig. 2). Meanwhile, participants with reticulation increased from 19 (7.0%) to 57 (21.0%). The ARDS pattern in three participants (1.1%) and crazy paving pattern in two participants (0.7%) at initial CT had disappeared at 6-month follow-up CT. Participants with organizing pneumonia pattern increased from four (1.5%) to seven (2.6%). Among CT evidence of fibrotic-like changes, participants with linear atelectasis increased from four (1.5%) to seven (2.6%) (Fig. 3), participants with bronchiectasis and parenchymal bands increased from six (2.2%) to 31 (11.4%) (Fig. 4) and 14 (5.2%) (Fig. 5) respectively. There was no change in the three participants (1.1%) with honeycombing. In summary, 39 participants (14.4%) demonstrated new suspicious fibrotic-like changes at 6-month follow-up CT.
Serial chest CT scans in a 45-year-old man with severe coronavirus disease 2019 pneumonia. (A, B) Initial CT scans obtained on day 5 after the onset of symptoms showed extensive ground-glass opacities (GGO) with some areas of consolidation bilaterally. (C, D) CT scans obtained on day 9 showed extensive consolidation with few GGOs bilaterally. (E, F) CT scans obtained on day 179 showed almost absorption of the abnormalities with mild GGOs and interstitial thickening remaining.
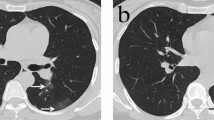
Serial chest CT scans in a 61-year-old man with coronavirus disease 2019 pneumonia. (A, B) Initial CT scans obtained on day 4 after the onset of symptoms showed multiple ground-glass opacities and consolidation bilaterally. (C) CT scans obtained on day 22 showed moderate consolidation and reticulation in the lower lung lobes bilaterally. (D) CT scans obtained on day 191 showed obviously absorption of the abnormalities with subtle reticulation and linear atelectasis (arrow) in the lower lung lobes.
Serial chest CT scans in a 60-year-old man with coronavirus disease 2019 pneumonia. (A, B) Initial CT scans obtained on day 8 after the onset of symptoms showed multiple ground-glass opacities and interstitial thickening bilaterally. (C, D) CT scans obtained on day 180 showed traction bronchiectasis (white arrow) and interlobar pleural traction (black arrow) in the upper lobe of right lung.
Serial chest CT scans in a 54-year-old man with coronavirus disease 2019 pneumonia. (A) Initial CT scans obtained on day 9 after the onset of symptoms showed multiple ground-glass opacities and interstitial thickening bilaterally. (B)CT scans obtained on day 169 showed traction bronchiectasis (white arrow) and parenchymal bands (black arrow) in the lower lung lobes.
Comparison of chest CT scores
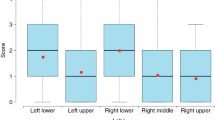
In the Chest CT scores (Table 3), a significantly decrease was found for any abnormality (P < 0.001), GGO (P < 0.001), and consolidation (P < 0.001), whereas a significantly increase for fibrotic-like abnormalities (P < 0.001) compared with the initial CT scans. Meanwhile, reticulation showed insignificantly change between two CT scans (P = 0.33).
Factors associated with pulmonary residual abnormalities
In the univariate analysis, paxlovid (odd ratio [OR]: 0.08; 95% CI 0.03, 0.21; P < 0.001), invasive ventilation (OR 9.3; 95% CI 2.8, 29; P < 0.001), age > 60 years (OR 6.5; 95% CI 2.7, 17; P < 0.001), SaO2 less than 93% at admission (OR 4.5; 95% CI 1.4, 14; P < 0.001), hospitalization more than 15 days (OR 3.8; 95% CI 1.3, 11; P = 0.002), and respiratory rate more than 23 times per minute at admission (OR 3.3; 95% CI 1.3, 8.7; P = 0.004) were associated with pulmonary residual abnormalities at 6-month follow-up CT. In the multivariate analysis, the predictive factors were invasive ventilation (OR 13.6; 95% CI 1.9, 45; P < 0.001), age > 60 years (OR 9.1; 95% CI 2.3, 39; P = 0.01), paxlovid (OR 0.11; 95% CI 0.04, 0.48; P = 0.01), hospitalization more than 15 days (OR 6.1; 95% CI 1.2, 26; P = 0.002), heart rate greater than 100 times per minute (OR 5.9; 95% CI 1.1, 27; P = 0.03), and SaO2 less than 93% at admission (OR 5.6; 95% CI 1.4, 13; P = 0.02) (Table 4).
Factors associated with pulmonary fibrotic-like changes
In the univariate analysis, paxlovid (OR 0.11; 95% CI 0.04, 0.32; P < 0.001), invasive ventilation (OR 8.8; 95% CI 2.1, 26; P < 0.001), smoker (OR 7.4; 95% CI 3.0, 16; P < 0.001), SaO2 less than 93% at admission (OR 4.5; 95% CI 1.2, 16; P = 0.002) and age > 60 years (OR 4.2; 95% CI 1.3, 11; P = 0.002) were associated with pulmonary fibrotic-like changes at 6-month follow-up CT. In the multivariate analysis, the predictive factors were invasive ventilation (OR 10.3; 95% CI 2.9, 33; P = 0.002), smoker (OR 9.9; 95% CI 2.4, 31; P = 0.01), paxlovid (OR 0.1; 95% CI 0.03, 0.48; P = 0.01), SaO2 less than 93% at admission (OR 7.8; 95% CI 1.5, 19; P = 0.02), age > 60 years (OR 6.1; 95% CI 2.3, 22; P = 0.03) and heart rate greater than 100 times per minute (OR 4.9; 95% CI 1.7, 11; P = 0.04) (Table 5).
Discussion
We prospectively followed COVID-19 pneumonia patients discharged from hospitals in China during the turn of 2022–2023 with chest CT to better understand the radiologic change with time. In several follow-up studies of COVID-19 pneumonia patients infected in 2020, the results indicated that 48–78% of the patients still had pulmonary abnormalities at 6-month follow-up5,17,18,19. A study of COVID-19 patients infected from February to May 2021 found that at least 66% of patients still had pulmonary abnormalities at 6-month follow-up CT, but the mean age of the patients were 82.3 years ± 7.1 (SD)20. With the worldwide phasing out of universal SARS-CoV-2 polymerase chain reaction test for the population until the increase of COVID-19 cases in China at the end of 2022, no new follow-up studies have been reported. In our study, 98 of 271 participants (36.2%) had radiographic abnormalities at the 6-month follow-up, with the most common findings being GGO (66 of 271 participants, 24.4%), and reticulation (57 of 271 participants, 21%). Our study showed the proportion of patients having residual pulmonary abnormalities in 6-month CT follow-up decreases after 2–3 years of genetic mutation of SARS-CoV-2. However, the most common pulmonary abnormalities were also GGO and reticulation, which did not differ from several of the studies mentioned above.
Pulmonary fibrosis is one of the most important concerns in the long-term follow-up of CT in COVID-19. Most studies judged pulmonary fibrosis based on the interpretation of the Fleischner Society glossary for CT features. Linear atelectasis, traction bronchiectasis, parenchymal banding and honeycombing are the most common features used to evaluate pulmonary fibrosis. Several studies have reported pulmonary fibrosis and(or) fibrotic-like changes in 35–56% of COVID-19 patients in 6-month CT follow-up7,20,21. In our study, 39 of 271 participants (14.4%) had fibrotic-like changes at the 6-month follow-up CT. The presence of fibrotic-like changes was reduced in our study compared to studies mentioned above. However, the selection and recognition of the relevant CT features differed somewhat in diverse studies. Most of the fibrotic-like changes in COVID-19 patients would disappear up to 1 year post hospital discharge21,22,23. It has been reported that in survivors of the preceding SARS pandemic, fibrotic-like changes present at 6 months may still improve at 84 months of follow-up, such as traction bronchiectasis24. Actually, periods of fibroproliferation of variable severity are part of the natural history of diffuse alveolar damage25,26. Most fibrotic-like changes are reversible, so it should be cautious to describe them as fibrotic changes or pulmonary fibrosis. The exact proportion of patients with long-term, irreversible fibrotic changes is unknown, but appears to be low, and the proportion of progressive pulmonary fibrosis is expected to be even lower, thus long-term follow-up studies are necessary27.
In both multivariable analyses of residual pulmonary abnormalities and pulmonary fibrotic-like changes, invasive ventilation ranked first among the risk factors. First, these patients were admitted to the intensive care unit, reflecting the severity of the disease and the slow recovery. In addition, some studies have confirmed that medically induced injury from invasive mechanical ventilation is probably an important cause of pulmonary fibrosis26,28. Paxlovid is another important independent prognostic factor, and it has been proven to be a very effective antiviral drug against SARS-CoV-229,30,31. Paxlovid, azvudine and glucocorticoid were the three most widely used drugs in the medication of the COVID-19 pneumonia in Beijing epidemic. Azvudine is another effective antiviral drug, which can increase the rate of nucleic acid negative conversion and early hospital discharge32,33. Glucocorticoid is effective in reducing mortality in severe COVID-19 patients34,35. However, in this study, using of Azvudine or glucocorticoid at the time of infection was not found to be effective in improving pulmonary residual abnormalities and fibrotic-like changes at 6-month follow-up. A reason for this may be that as an antiviral drug, the effect of Azvudine in inhibiting SARS-CoV-2 is weaker than that of Paxlovid, which was confirmed in a recent study36. Glucocorticoid acts to reduce the inflammatory response in the acute phase37, and may not relieve lung lesions in the chronic phase.
The long-term follow-up of COVID-19 is necessary but will inevitably increase considerable amount of work. Artificial intelligence (AI) may be helpful in detecting lung abnormalities and improving efficiency. AI models help in visualization and measurement of COVID-19 specific lesions in the lungs of infected patients, potentially facilitating timely, patient-specific medical interventions38. Moreover, AI has a good correlation with radiologists in detecting lung lesions, but more efficiently39.
This study has some limitations. First, this study did not correlate the performance of CT with clinical symptoms, laboratory examinations, and especially abnormalities in lung function, which was greatly restricted during the epidemic due to infectious disease control and prevention, and therefore could not be compared with lung function results at follow-up. However, changes in lung function play an important role in evaluating the regression of COVID-19 pneumonia and interstitial fibrosis in patients40. So, lack of relevant data makes a limitation of our study. Second, at the beginning of the COVID-19 epidemic in Beijing, due to the lack of paxlovid, some patients did not start paxlovid until the 2nd–4th day after admission to the hospital. Gradually, patients were able to ensure that standardized paxlovid therapy as soon as they were admitted to the hospital. This difference may have had some impact on the results. However, even so, the results suggest that paxlovid may has a promising effect on the long-term regression of the COVID-19 pneumonia.
In conclusion, this prospective study showed that in the COVID-19 pandemic of China during the turn of 2022–2023, a considerable proportion of the patients with pulmonary residual abnormalities and fibrotic-like changes were found at the 6-month follow-up CT. Furthermore, it was observed that antivirals against SARS-CoV-2 like paxlovid may be beneficial for long-term regression of COVID-19. While it is commonly assumed that the genetic mutations of SARS-CoV-2 have led to a significant decrease in its pathogenicity, our study indicates the importance of long-term follow-up for patients with COVID-19 pneumonia.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus type 2
- GGO:
-
Ground-glass opacity
- ARDS:
-
Acute respiratory distress syndrome
References
Guan, W. J. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 382(18), 1708–1720 (2020).
Hu, B., Guo, H., Zhou, P. & Shi, Z. L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 19(3), 141–154 (2021).
Rubin, G. D. et al. The role of chest imaging in patient management during the COVID-19 pandemic: A multinational consensus statement from the fleischner society. Chest. 158(1), 106–116 (2020).
Kwee, T. C. & Kwee, R. M. Chest CT in COVID-19: What the radiologist needs to know. Radiographics. 40(7), 1848–1865 (2020).
Wu, X. et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: A prospective study. Lancet Respir Med. 9(7), 747–754 (2021).
Huang, L. et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet. 398(10302), 747–758 (2021).
Bocchino, M. et al. Chest CT-based assessment of 1-year outcomes after moderate COVID-19 pneumonia. Radiology. 305(2), 479–485 (2022).
Walensky, R. P., Walke, H. T. & Fauci, A. S. SARS-CoV-2 variants of concern in the United States-challenges and opportunities. JAMA. 325(11), 1037–1038 (2021).
Tao, K. et al. The biological and clinical significance of emerging SARS-CoV-2 variants. Nat Rev Genet. 22(12), 757–773 (2021).
Tsakok, M. T. et al. Reduction in chest CT severity and improved hospital outcomes in SARS-CoV-2 omicron compared with delta variant infection. Radiology. 306(1), 261–269 (2023).
Wolter, N. et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: A data linkage study. Lancet. 399(10323), 437–446 (2022).
Zhang L, Zhang Y, Duan W, et al. Using an influenza surveillance system to estimate the number of SARS-CoV-2 infections in Beijing, China, weeks 2 to 6 2023. Euro Surveill. 2023. 28(11).
Leung, K., Lau, E., Wong, C., Leung, G. M. & Wu, J. T. Estimating the transmission dynamics of SARS-CoV-2 Omicron BF.7 in Beijing after adjustment of the zero-COVID policy in November-December 2022. Nat Med. 29(3), 579–582 (2023).
Hansell, D. M. et al. Fleischner society: Glossary of terms for thoracic imaging. Radiology. 246(3), 697–722 (2008).
Antonio, G. E. et al. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: Preliminary experience. Radiology. 228(3), 810–815 (2003).
Francone, M. et al. Chest CT score in COVID-19 patients: Correlation with disease severity and short-term prognosis. Eur Radiol. 30(12), 6808–6817 (2020).
Luger, A. K. et al. Chest CT of lung injury 1 year after COVID-19 Pneumonia: The CovILD study. Radiology. 304(2), 462–470 (2022).
Han, X. et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 299(1), E177–E186 (2021).
Huang, C. et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 397(10270), 220–232 (2021).
Di Pentima C, Cecchini S, Spannella F, et al. Radiological lung sequelae, functional status and symptoms in older patients 3 and 6 months after hospitalization for COVID-19 pneumonia. Intern Emerg Med. 2023, pp 1–11
Han, X. et al. Fibrotic interstitial lung abnormalities at 1-year follow-up CT after severe COVID-19. Radiology. 301(3), E438–E440 (2021).
Vijayakumar, B. et al. CT lung abnormalities after COVID-19 at 3 months and 1 year after hospital discharge. Radiology. 303(2), 444–454 (2022).
Pan, F. et al. Chest CT patterns from diagnosis to 1 year of follow-up in patients with COVID-19. Radiology. 302(3), 709–719 (2022).
Wu, X., Dong, D. & Ma, D. Thin-section computed tomography manifestations during convalescence and long-term follow-up of patients with severe acute respiratory syndrome (SARS). Med Sci Monit. 22, 2793–2799 (2016).
Marshall, R. P. et al. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med. 162(5), 1783–1788 (2000).
Desai, S. R., Wells, A. U., Rubens, M. B., Evans, T. W. & Hansell, D. M. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology. 210(1), 29–35 (1999).
Kuo, M. D. et al. Multi-center validation of an artificial intelligence system for detection of COVID-19 on chest radiographs in symptomatic patients. Eur Radiol. 33(1), 23–33 (2023).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 395(10223), 497–506 (2020).
Wong, C. et al. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: An observational study. Lancet. 400(10359), 1213–1222 (2022).
Dryden-Peterson S, Kim A, Kim AY, et al. Nirmatrelvir plus ritonavir for early COVID-19 and hospitalization in a large US health system. medRxiv. 2022.
Malden, D. E. et al. Hospitalization and emergency department encounters for COVID-19 after paxlovid treatment—California, December 2021–May 2022. MMWR Morb Mortal Wkly Rep. 71(25), 830–833 (2022).
Zhang, J. L. et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct Target Ther. 6(1), 414 (2021).
Ren, Z. et al. A Randomized, open-label, controlled clinical trial of Azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv Sci (Weinh). 7(19), e2001435 (2020).
WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA. 324(13), 1330–1341 (2020).
Monedero, P. et al. Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: A cohort study. Crit Care. 25(1), 2 (2021).
Gao, Y. et al. Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19. J Infect. 86(6), e158–e160 (2023).
RECOVERY Collaborative Group et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 384(8), 693–704 (2021).
Roth HR, Ziyue X, Carlos T, et al. Rapid artificial intelligence solutions in a pandemic—The COVID-19-20 Lung CT Lesion Segmentation Challenge. Med. Image Anal. 2022. 82.
Liang, H. et al. Artificial intelligence for stepwise diagnosis and monitoring of COVID-19. Eur Radiol. 32(4), 2235–2245 (2022).
Han, X. et al. Longitudinal assessment of chest CT findings and pulmonary function after COVID-19 infection. Radiology. 307(2), e222888 (2023).
Acknowledgements
Lv Yuan has been working in the temporary COVID-19 ward in the turn of 2022–2023.
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.Z. and X.F.; methodology, Y.Z.; software, Y.L.; validation, X.F. and Y.L.; formal analysis, W.L. and L.L (Lin Liu).; investigation, Y.F.; resources, Y.F.; data curation, L.L (Li Liu) and F.P.; writing—original draft preparation, X.F.; writing— review and editing, Y.Z. and Y.L.; visualization, X.F.; supervision, Y.Z.; project administration.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fang, X., Lv, Y., Lv, W. et al. CT-based Assessment at 6-Month Follow-up of COVID-19 Pneumonia patients in China. Sci Rep 14, 5028 (2024). https://doi.org/10.1038/s41598-024-54920-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-54920-1