Abstract
The COVIVAX study assessed the association between COVID-19 vaccination and the risk of common neurological disorders in a multicenter case-control design. Vaccination exposure was compared between individuals with a first diagnosis of a neurological disorder (cases) and age- and sex-matched controls. A total of 624 participants were enrolled, and after random 1:1 matching 265 cases and 265 matched controls (total 530 participants) were included in the analyses. The most frequent neurological diagnosis in cases were stroke (60.4%), multiple sclerosis (11.3%) and seizures (6.4%). The proportion of vaccinated participants was 72.1% among cases and 79.6% among controls. A protective role of vaccination on the risk of developing a new neurological disorder was detected in the unadjusted analysis (OR 0.50; 95% CI 0.29–0.86; p = 0.0114). After adjustment for confounders, the number of vaccination doses received was associated with a reduced risk of developing new neurological disorders for participants aged over 60 years ( p = 0.0472; OR 0.14, 95% CI 0.03–0.68), with pre-existing comorbidities (p = 0.0122; OR 0.04, 95% CI 0.01–0.99) and for stroke (p = 0.0232; OR 0.04, 95% CI 0.02–0.97). The COVIVAX study provided no warning sign regarding an increase in the risk of developing new neurological disorders following COVID-19 vaccination of any type or doses. A potentially protective effect of multiple doses of COVID-19 vaccines against the risk of stroke in people aged over 60 needs to be confirmed by further studies.
Similar content being viewed by others
Introduction
A number of neurological symptoms, signs and diseases have been reported during the acute and post-acute phase of COVID-19 infection1,2,3,4,5,6,7,8,9,10,11. Some minor symptoms, such as headache or myalgia, could be interpreted as part of the general symptoms of the viral infection. However, more serious neurological conditions, such as acute ischemic stroke, Guillain-Barrè syndrome or seizures, have been associated to either thrombo-inflammation or immune-mediated mechanisms caused by the host response to the virus, rather than by direct viral injury to the central nervous system12,13,14,15.
The rapid development and widespread use of COVID-19 vaccines represented a remarkable, and unprecedented, turning point of this viral pandemic, at a global level. The vaccination campaigns, performed over less than 1 year in 2021 in many developed countries, largely prevented death and morbidity associated with COVID-19 infection16,17. Safety of COVID-19 vaccines have been carefully evaluated during clinical trials and post-marketing surveillance18. In particular, the specific risk of vaccine-associated neurological disorders have been assessed in self-controlled studies and appeared to be low19. However, a dedicated case-control study on the casual association between neurological disorders and exposure to COVID-19 vaccines is lacking.
The aim of the COVIVAX study was to investigate the relative risk of developing common neurological disorders in participants exposed versus those not exposed to COVID-19 vaccines, using a multicentre, case-control design.
Results
Study population
A total of 303 cases and 321 controls were included in the study, and among them 265 pairs were randomly matched by age and sex. The median time from first dose of COVID vaccination and diagnosis was 3.5 months (IQR 2.0-5.8 months, range 1.0-11.4 months). The main clinical characteristics of the matched cases are shown in Table 1. The most represented neurological disease was stroke (n = 160, 60.4%), followed by multiple sclerosis (n = 30, 11.3%) and epilepsy/seizures (n = 17, 6.4%). The mean age at diagnosis was 64 years (interquartile range (IQR) 50–74). The majority of cases (81%) were enrolled in hospital and 19% from outpatient services. Demographics and comorbidities of cases and matched controls are shown in Table 2. Males were slightly predominant (53%) in both groups and the median age at interview was 65 years (IQR 52–75). A significant difference in years of education between cases and controls was observed, with cases showing less years of education than matched controls. More than one half of the sample had at least one comorbidity in both groups. The prevalent comorbidities were cardiovascular (42.6% of cases and 33.6% of controls), followed by metabolic among cases (16.2%) and bone diseases among controls (11.3%).
Relative risk of neurological disorders in vaccinated and unvaccinated participants
At least one dose of any COVID-19 vaccine was received by 72.1% of cases and 79.6% of controls, with a statistically significant reduction in the risk of developing neurological diseases in vaccinated participants, compared to unvaccinated participants (OR 0.50; 95% CI 0.29–0.86, p = 0.0114). However, the risk reduction was not confirmed by multivariable analysis, after adjustment for comorbidities, education and exposure to COVID-19 or flu infection (Table 3). When considering the number of vaccine doses a significant risk reduction was observed with increasing number of doses: the risk reduction was not significant in participants receiving only one dose (OR 0.68; 95% CI 0.35–1.30), while significant risk reductions were observed for 2 (OR 0.40; 95% CI 0.22–0.72) and 3 or more doses (0.18; 95% CI 0.06–0.52). This effect was also not confirmed by multivariable analysis (Table 3). No significant effects were observed for vaccine type or exposure to COVID-19 or flu (Table 3). In subgroup analyses, a significant effect of the number of doses of vaccine in reducing the risk of neurological diseases were observed in participants aged over 60 years, in those with a diagnosis of stroke, in females and in those with comorbidities (Table 4). The significance of this association was confirmed by multivariable analysis only for participants aged over 60 ( p = 0.0472; OR 0.14, 95% CI 0.03–0.68), with pre-existing comorbidities (p = 0.0122; OR 0.04, 95% CI 0.01–0.99) and with a diagnosis of stroke (p = 0.0232; OR 0.04, 95% CI 0.02–0.97).
Initial severity and long-term neurological outcome of neurological disorders
No differences in the frequency of vaccination was found between cases who had a worst functional outcome at diagnosis (mRS = 2+) and those with less disability (mRS = 0–1) (Supplemental Table S1). Cases with 12-month follow-up were 274, among them 114 (41.6%) showed an improvement in the mRS score at follow-up as compared to the time of discharge, 146 (53.3%) were stable and 14 (5.1%) worsened. A diagnosis of stroke was the most frequent among cases who improved or remained stable, followed by multiple sclerosis and epilepsy/seizures, while among worsened cases the most represented were Parkinson’s disease/Parkinsonisms and motor neuron diseases (Supplemental Table S2). No difference in the frequency of vaccination was found between cases who improved, were stable or worsened in the entire sample, nor by specific neurological diagnosis (Supplemental Table S3).
Discussion
The COVIVAX study investigated the association between exposure to COVID-19 vaccines and the risk of common neurological disorders, using a case-control study design. The frequency of newly diagnosed neurological disorders (approximately 60% stroke, 11% multiple sclerosis, 6% epilepsy) and vaccinated versus unvaccinated participants (approximately 75% versus 25%) reflected the predominantly hospital-based recruitment and the timing of the study conduction with regards to the COVID-19 vaccination campaign.
Our findings clearly indicate that exposure to COVID-19 vaccines, regardless of type or number of doses, does not increase the risk of newly diagnosed common neurological disorders, in particular stroke, multiple sclerosis and epilepsy. Conversely, our results suggested a potentially protective effect of COVID-19 vaccination against stroke in persons aged over 60 and with comorbidities who received multiple doses of vaccine. This finding should be interpreted with caution, given the limited sample size and the presence of confounding factors such as differences in education levels and a higher prevalence of cardiovascular comorbidities among cases. Despite these limitations, the multivariable analysis suggested a trend toward risk reduction in the overall population, with a significant risk reduction observed in high-risk subgroups. Our findings align with recent studies that demonstrate a reduction in overall cerebrovascular risk20, hospitalization from stroke21 and cerebral small vessel disease progression22 among individuals who received COVID-19 vaccination. Further research with larger cohorts is warranted to corroborate these findings and elucidate the underlying mechanisms driving this protective effect.
No difference in disease-related disability at hospital discharge was observed between vaccinated and unvaccinated participant, indicating no sign of increased disease severity associated with COVID-19 vaccination. Moreover, over 90% of cases were followed for 12 months and the course of neurological disorders was not different in either vaccinated or unvaccinated participants.
Traditional case-controlled studies have the advantages of being more accurate for cumulative exposures (e.g. multiple dose of vaccine) and disorders with increased mortality (e.g. stroke), compared to self-controlled studies23. The results of two previous, large studies specifically focused on neurological safety of COVID-19 vaccines are available, both of them used a self-controlled study design, routinely collected or administrative data as data sources and were mostly focused on immune-mediated neurological disorders. A first, self-controlled study including over 30 million people who received only the first dose of ChAdOx1nCoV-19 or BNT162b2 estimated a slightly increased risk of Guillain-Barré syndrome (38 excess cases per 10 million people) after receiving the ChAdOx1nCoV-19 vaccine and a possibly increased risk of hemorrhagic stroke (60 excess cases per 10 million people; however, this magnitude was reduced in sensitivity analyses that accounted for fatal events) after received the BNT162b2 vaccine24. A second, self-controlled study including over 8 million people who received at least one dose of COVID-19 vaccines (ChAdOx1 nCoV-19, BNT162b2, mRNA-1273, or Ad.26.COV2.S) showed no higher than expected rates of immune-mediated neurological disorders, in particular Bell’s palsy, encephalomyelitis, and Guillain-Barré syndrome25.
Our study has strengths and limitations. Cases were exclusively recruited by neurologists who were involved in patient’s management. This neurologist-centered approach of patient ascertainment had the major advantage of high standards of diagnostic accuracy and disease management, which was guaranteed by specialized medical professionals, with direct consequences on disease definitions and data collection, compared to administrative data. The current World Health Organization definition of adverse events following immunization (AEFI) provides broad temporal criteria for neurological disorders, ranging from 1 week to 1 year, which is consistent with the timing investigated in the COVIVAX study26. Moreover, the cases recruited in the COVIVAX study reflected the epidemiology of newly diagnosed common neurological disorders, such as stroke, multiple sclerosis and epilepsy. Data from age- and sex-matched controls have not been collected by previous studies, thus the COVIVAX study added methodological strength and novelty to the previous evidence available on this topic.
A major limitation was the limited sample size, which allowed us to estimate the attributable risk only for common neurological disorders, such as stroke, multiple sclerosis or epilepsy. Other rarer neurological conditions were under-represented in our study, in particular immune-mediated disorders such as Guillain-Barrè syndrome or encephalitis, and our results were not powered to make any inference about them. However, this study was performed during the pandemic (year 2021), while the Italian health system was still considerably under pressure. This led the Investigators to collect a limited number of cases and matched controls.
Another major limitation is that the study did not reach the pre-planned sample size of 410 cases and 410 controls, only 365 cases and 365 matched controls were recruited. However, the sample size calculation was based on the assumption that only 40% of the controls would have been vaccinated, while 79.6% were actually vaccinated, and also in the planning phase a risk increase was hypothesized while a risk reduction was then observed. The results of the study should be interpreted in light of these limitations.
The COVIVAX study provided no warning sign regarding an increased risk of developing new neurological disorders, in particular stroke and multiple sclerosis, following COVID-19 vaccination of any type or doses.
A potentially protective effect of multiple doses of COVID-19 vaccines against the risk of stroke in people aged over 60 and with comorbidities was unexpected, needs to be confirmed by further studies and might affect public health policies at a global level.
Methods
Study design
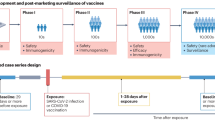
The COVIVAX study was a multicentre, observational case-control study performed in seven Neurology departments (including inpatient and outpatient facilities) in Italy.
Eligible participants (cases) were consecutive individuals with a new diagnosis of one of the following diseases: stroke, Alzheimer’s disease or other dementias, Parkinson’s disease or Parkinsonism, motor neuron disease, epilepsy, multiple sclerosis, myelitis or non-traumatic myelopathy, Guillain Barrè syndrome or other peripheral neuropathies, myopathies of unknown origin, cerebral venous thrombosis. Participants in whom the target neurological diseases are pre-existent, with unknown medical history or not giving informed consent were excluded.
Controls were individuals not related to cases and with none of the target neurological diseases.
Cases and controls were then 1:1 randomly matched by age (± 5 years) and by sex. Only matched cases and controls were included in subsequent analyses.
Cases were considered exposed to COVID-19 vaccine if they received at least one dose before diagnosis. The exposure to multiple doses was also evaluated considering only the period before diagnosis. Controls were matched to cases at the time of the diagnosis of their matched case and exposures were evaluated before the date of diagnosis of their matched case.
The primary outcome of the study was the relative risk of new neurological disorders in participants exposed to COVID-19 vaccine (vaccinated) versus not exposed (unvaccinated) and was estimated as an odds ratio (OR). Secondary outcomes included: (1) the relative risk of new neurological disorders in selected subpopulations (age over 60, individuals with comorbidities) in vaccinated versus unvaccinated participants; (2) the relative risk of selected neurological diseases (stroke, neurodegenerative diseases, immune-mediated diseases) in vaccinated versus unvaccinated participants. (3) neurological disability, measured by the modified Rankin scale, at discharge (dichotomized as 0–1 versus 2 or more) and at 12-month follow-up (assessed as improved, stable or worsened, compared to discharge), in vaccinated versus unvaccinated cases.
Ethics and data collection
The study protocol was approved by the Ethics Committee of Spallanzani Institute, Rome, which is responsible for all clinical studies regarding COVID-19 vaccines in Italy. The study conforms with World Medical Association Declaration of Helsinki. All participants gave written informed consent.
The study started on May 1st, 2021. Eligible cases were identified retrospectively among consecutive patients admitted to the hospital or seen in the outpatient services of the participating centers from January to December 31st, 2021. Each recruiting site was asked to recruit controls, chosen among relatives and friends not hospitalized of the corresponding cases. A subsequent follow-up of cases was performed after 12 months from the diagnosis to evaluate disease progression. Participants (both cases and controls) were interviewed by a medical professional qualified in the diagnosis and treatment of neurological diseases (either a Neurology Consultant or a Neurology Resident) during an outpatient visit or a phone call. The interview aimed to collect demographic and clinical variables, including data on exposure to COVID-19.
Comorbidities were classified according to a selection of the MedDRA organ/system categories, as follows: cardiac or vascular disorders, nervous system disorders (other than target), respiratory disorders, gastrointestinal disorders, renal, urinary or reproductive system disorders, immune system disorders, musculoskeletal and connective tissue disorders, blood and lymphatic system disorders, endocrine disorders, metabolic and nutrition disorders, neoplasms27.
COVID-19 symptoms were defined as a clinical diagnosis of COVID by any physician, based on COVID symptoms of any severity and a positive antigenic or molecular COVID test.
Flu symptoms were defined as a clinical diagnosis of flu by any physician, based on typical flu symptoms and a negative antigenic or molecular COVID test.
The structure of the interview was the same for cases and controls (see Supplementary Material).
An electronic case-report form (e-CRF) was prepared using a centralized password protected database Guideline FDA, cfr21 paragraph 11 Compliant, performed by Advice Pharma Group S.r.l.
Statistical analysis
Descriptive statistics were performed in cases with neurological diseases and matched controls on all demographic and clinical variables. Categorical variables were reported as frequencies with percentage, while numerical variables as medians with interquartile range. Cases and matched controls were compared using the Chi-square or the Fisher’s exact test for categorical variables, and the Wilcoxon-Mann-Whitney test for numerical variables. The association between vaccine and neurological diseases was evaluated with univariable and multivariable conditional logistic regression models with the group (case or control) as dependent variable and vaccine as independent variable. Multivariable models were adjusted for variables that were considered as potential confounders: education, comorbidities, history of COVID-19, history of flu. Different models were used to assess the effect of different vaccine doses and vaccine types on the risk of developing neurological diseases, considering the following as independent variables: at least one dose of any COVID-19 vaccine (yes, no); number of COVID-19 vaccine doses (0, 1, 2, 3+); at least one dose of BNT162b2 (Pfizer/BioNTech®) (yes, no); at least one dose of mRNA-1273 (Moderna®) (yes, no); at least one dose of ChAdOx1 nCoV-19 (AstraZeneca®) (yes, no); at least one dose of Ad.26.COV2.S (Janssen®) (yes, no). The effect of COVID-19 symptoms and flu symptoms on the risk of developing neurological diseases were also assessed in univariable and multivariable conditional logistic regression models. Results were reported as Odds Ratios (OR) and adjusted Odds Ratios (adj. OR) with 95% Confidence Intervals (95% CI). Analyses evaluating the effect of having received at least one dose of any vaccine, the number of doses and the exposure to COVID-19 were also evaluated in subgroups defined according to age (≤ 60 years, > 60 years), sex, presence of any pre-existing comorbidity (no comorbidities, at least 1 comorbidity), neurological disease (only diseases with at least 15 cases identified).
The risk of developing neurological diseases with higher levels of functional disability was assessed in cases by comparing counts and percentages of vaccinated and unvaccinated between those with mRS = 0–1 versus mRS = 2 + using the Chi-square or the Fisher’s exact test. Additional analyses were conducted including only cases with an available follow-up at 12 months after the diagnosis of their neurological condition. Cases were classified as improved, stable or worsened according to their modified Rankin Scale (mRS) score at the 12-month follow-up compared to the score at discharge: improved were those with an mRS score at 12 months that was lower than the score at discharge; stable were those with mRS score at 12 months that was equal to the score at discharge; worsened were those with a score at 12 months that was higher than the score at discharge. Descriptive statistics were reported separately in improved, stable and worsened cases and the three groups were compared with the chi-square or the Fisher’s exact test for categorical variables and the Kruskal-Wallis test for numerical variables. The count and percentage of cases who received at least one dose of COVID-19 vaccine by functional status (improved, stable, worsened) at the 12-month follow-up were reported in the entire sample of cases with 12-month follow-up available and separately by each neurological diagnosis.
All analyses were conducted using the SAS software, version 9.4 (SAS Institute, Cary, NC, USA). Significance level was set at 5% (p = 0.05). No missing data were reported for variables that were included in the analyses.
Sample size calculation
Assuming that by the end of the study about 75% of the Italian population would have been vaccinated, starting from a 10% at the beginning of the study, with an 8% absolute increase per month, the mean expected proportion of vaccinated participants over the entire study period among the controls was estimated to be about 40%. In order to detect an odds ratio of 1.5 (proportion of vaccinated among cases about 50%) with 80% power and a 5% level of significance, a total of 410 cases and 410 controls was estimated to be required.
Data availability
Investigators may request access to anonymized individual patient data and trial documents including raw datasets, analysis-ready datasets, trial protocols, annotated case report form, statistical analysis plan and dataset specifications by contacting the corresponding author. Prior to use of the data, proposals need to be approved by the COVIVAX Steering Committee and a signed data sharing agreement will then be approved.
References
Mao, L. et al. Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 77, 683–690. https://doi.org/10.1001/jamaneurol.2020.1127 (2020).
Chen, T. et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj 368, m1091. https://doi.org/10.1136/bmj.m1091 (2020).
Ellul, M. A. et al. Neurological associations of COVID-19. Lancet Neurol. 19, 767–783. https://doi.org/10.1016/s1474-4422(20)30221-0 (2020).
Collantes, M. E. V., Espiritu, A. I., Sy, M. C. C., Anlacan, V. M. M. & Jamora, R. D. G. Neurological manifestations in COVID-19 infection: a systematic review and Meta-analysis. Can. J. Neurol. Sci. 48, 66–76. https://doi.org/10.1017/cjn.2020.146 (2021).
Di Carlo, D. T. et al. Exploring the clinical association between neurological symptoms and COVID-19 pandemic outbreak: a systematic review of current literature. J. Neurol. 268, 1561–1569. https://doi.org/10.1007/s00415-020-09978-y (2021).
Romero-Sánchez, C. M. et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology 95, e1060–e1070. https://doi.org/10.1212/wnl.0000000000009937 (2020).
Romoli, M. et al. A systematic review of neurological manifestations of SARS-CoV-2 infection: the devil is hidden in the details. Eur. J. Neurol. 27, 1712–1726. https://doi.org/10.1111/ene.14382 (2020).
Herman, C., Mayer, K. & Sarwal, A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID-19. Neurology 95, 77–84. https://doi.org/10.1212/wnl.0000000000009673 (2020).
Leonardi, M., Padovani, A. & McArthur, J. C. Neurological manifestations associated with COVID-19: a review and a call for action. J. Neurol. 267, 1573–1576. https://doi.org/10.1007/s00415-020-09896-z (2020).
Pezzini, A. & Padovani, A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 16, 636–644. https://doi.org/10.1038/s41582-020-0398-3 (2020).
Beretta, S. et al. Incidence and long-term functional outcome of neurologic disorders in hospitalized patients with COVID-19 infected with Pre-omicron variants. Neurology 101, e892–e903. https://doi.org/10.1212/wnl.0000000000207534 (2023).
Iba, T., Connors, J. M. & Levy, J. H. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm. Res. 69, 1181–1189. https://doi.org/10.1007/s00011-020-01401-6 (2020).
Zhou, Z., Kang, H., Li, S. & Zhao, X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 267, 2179–2184. https://doi.org/10.1007/s00415-020-09929-7 (2020).
Zubair, A. S. et al. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of Coronavirus Disease 2019: a review. JAMA Neurol. 77, 1018–1027. https://doi.org/10.1001/jamaneurol.2020.2065 (2020).
Matschke, J. et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 19, 919–929. https://doi.org/10.1016/s1474-4422(20)30308-2 (2020).
Haas, E. J. et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 397, 1819–1829. https://doi.org/10.1016/s0140-6736(21)00947-8 (2021).
Agrawal, U. et al. COVID-19 hospital admissions and deaths after BNT162b2 and ChAdOx1 nCoV-19 vaccinations in 2·57 million people in Scotland (EAVE II): a prospective cohort study. Lancet Respir Med. 9, 1439–1449. https://doi.org/10.1016/s2213-2600(21)00380-5 (2021).
Jiesisibieke, Z. L., Liu, W. Y., Yang, Y. P., Chien, C. W. & Tung, T. H. Effectiveness and safety of COVID-19 vaccinations: an Umbrella Meta-Analysis. Int. J. Public. Health. 68, 1605526. https://doi.org/10.3389/ijph.2023.1605526 (2023).
Pottegård, A. & Klungel, O. H. The neurological safety of covid-19 vaccines. Bmj 376, o522. https://doi.org/10.1136/bmj.o522 (2022).
Chen, S. Y. et al. Prior COVID-19 vaccination and reduced risk of cerebrovascular diseases among COVID-19 survivors. J. Med. Virol. 96, e29648. https://doi.org/10.1002/jmv.29648 (2024).
Xiang, Y., Feng, Y., Qiu, J., Zhang, R. & So, H. C. Association of COVID-19 vaccination with risks of hospitalization due to cardiovascular and other diseases: a study using data from the UK Biobank. Int. J. Infect. Dis. 145, 107080. https://doi.org/10.1016/j.ijid.2024.107080 (2024).
Ip, B. et al. COVID-19 vaccination and cerebral small vessel disease progression - A prospective cohort study. Int. J. Infect. Dis. 107324 https://doi.org/10.1016/j.ijid.2024.107324 (2024).
Petersen, I., Douglas, I. & Whitaker, H. Self controlled case series methods: an alternative to standard epidemiological study designs. Bmj 354, i4515. https://doi.org/10.1136/bmj.i4515 (2016).
Patone, M. et al. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat. Med. 27, 2144–2153. https://doi.org/10.1038/s41591-021-01556-7 (2021).
Li, X. et al. Association between covid-19 vaccination, SARS-CoV-2 infection, and risk of immune mediated neurological events: population based cohort and self-controlled case series analysis. Bmj 376 (e068373). https://doi.org/10.1136/bmj-2021-068373 (2022).
(WHO), W. H. O. Causality assessment of an adverse event following immunization (AEFI): user manual for the revised WHO classification, 2nd ed., https://www.who.int/publications/i/item/9789241516990 (2019).
Giussani, G. et al. Comorbidities in patients with epilepsy: frequency, mechanisms and effects on long-term outcome. Epilepsia 62, 2395–2404. https://doi.org/10.1111/epi.17022 (2021).
Acknowledgements
S.B. and C.F. contributed to this work during their personal involvement in the Italian Ministry of University and Research (MUR) “Dipartimenti di Eccellenza 2023-2027”.
Funding
The study was funded by the by the Italian Society of Neurology (SIN).
Author information
Authors and Affiliations
Contributions
EP, EtB and SB conceived and designed the work.FB, AG, EDS, MC, CMC, GM, PC, GP, GF, AP, VC, AP, DA, SG, GT, AD, DRM, AG, AB, AS, PB, CS, LK, RV, GA, AB, SC, AZ, CF and SB contributed to data acquisiton.EP, ElB and SB contributed to data analysis and interpretation.EP and SB drafted and revised the workAll Authors approved the submitted version of the manuscript and agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pupillo, E., Bianchi, E., Beghi, E. et al. Multicentre case-control study on the association between COVID-19 vaccines and neurological disorders (COVIVAX). Sci Rep 15, 4179 (2025). https://doi.org/10.1038/s41598-025-88837-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-88837-0