Abstract
Purpose
This study aims to investigate the spectrum of viruses leading to severe viral pneumonia (SVP) and the associated risk factors for mortality among pediatric patients in the pediatric intensive care unit (PICU).
Methods
Taking the outbreak and end of the COVID-19 pandemic as a aboundary, The pre-pandemic period of COVID-19 spans from 01/2017 to 12/2019, the pandemic period from 01/2020 to 12/2021, and the post-pandemic period from 01/2022 to 12/2023. Patients were subsequently stratified into survivor and non-survivor groups based on clinical outcomes.
Results
A total of 1007 patients (median age 1.42 years, range 0.58–4.00; male: female ratio 1.7:1) diagnosed with SVP. Cases were stratified into pre-pandemic (n = 419, 41.6%), pandemic (n = 272, 27.0%), and post-pandemic (n = 316, 31.4%) periods. Viral predominance varied across phases: Pre-pandemic: Influenza A (IVA, 37.0% [155/419]), respiratory syncytial virus (RSV, 29.8%), adenovirus (19.8%), and influenza B (15.5%). Pandemic phase: Human rhinovirus (HRV, 40.1% [109/272]), RSV (33.1%), parainfluenza viruses (11.4%), and bocavirus (HBoV, 10.7%). Post-pandemic: HRV (24.4% [77/316]), RSV (22.8%), HBoV (14.2%), and IVA (13.6%). Comparative analysis revealed significant intergroup differences in the proportion of patients aged < 3 years, primary immunodeficiency disorders (PIDs), and sepsis between pure viral infection deaths and coinfection-associated fatalities among SVP cases. Logistic regression identified eight independent mortality predictors: acute leukemia, other malignant tumors, PIDs, moderate-to-severe underweight, rhabdomyolysis, acute respiratory distress syndrome (ARDS), infection-related encephalopathy, and multiorgan dysfunction syndrome (MODS). The prediction model demonstrated robust discriminative capacity for SVP mortality: sensitivity 73.8%, specificity 90.2%, and AUC 0.888 (95%CI 0.838–0.938) via ROC curve analysis.
Conclusions
The COVID-19 pandemic has altered the landscape of respiratory viruses causing SVP in children. The presence of underlying health conditions, particularly acute leukemia, other malignancies, and immunodeficiency, significantly increases the risk of death in children with viral pneumonia. The risk prediction model offers a reliable tool for clinical practice to predict mortality in these patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
1 Introduction
Pneumonia stands as a leading cause of child mortality, presenting a significant infectious threat to children’s health [1]. China has made remarkable strides in improving child survival of pneumonia, However, the proportion of children dying from pneumonia remains high at 13% [2]. The primary cause of pediatric respiratory illnesses is viruses. The emergence of the novel coronavirus, SARS-CoV-2, in December 2019 has precipitated a global health crisis marked by respiratory infections that have reverberated across both public health and economic domains. From May to December 2021, the widespread administration of the COVID-19 vaccine in China, significantly reducing the risk of infection and transmission [3]. However, the widespread implementation of anti-epidemic measures has markedly altered the pathogenic landscape of pediatric respiratory infections in pandemic. Despite the global impact of the COVID-19 pandemic, data characterizing the epidemiological trends of viral pneumonia pathogens in Chinese Pediatric Intensive Care Units across the pre-pandemic, pandemic, and post-pandemic periods remain scarce. This retrospective study analyzed clinical data from children with SVP admitted to a PICU, comparing pathogen profiles across pre-pandemic, pandemic, and post-pandemic phases of COVID-19 and identifying mortality-associated risk factors. By evaluating temporal variations in the SVP pathogen spectrum, this work aims to enhance clinical awareness among healthcare providers and identify risk factors associated with mortality in affected patients.
2 Objectives and Methods
2.1 Research Subjects
We conducted a retrospective analysis of clinical data from children afflicted with SVP, admitted to the PICU of Shenzhen Children’s Hospital from January 2017 to December 2023. Inclusion criteria required patients to meet the following conditions: (1) aged between 28 days and 14 years, (2) having laboratory confirmation of viral infection via pathogen testing of respiratory specimens, and (3) exhibiting clinical symptoms consistent with the diagnostic standards for severe pneumonia in children. The severity of pediatric pneumonia was classified based on predefined criteria [4]:
-
1)
Major criteria: Requirement for invasive mechanical ventilation, fluid-refractory shock, urgent noninvasive positive-pressure ventilation, or hypoxemia requiring FiO₂ exceeding general ward capacity.
-
2)
Minor criteria: Respiratory rate exceeding WHO age-specific thresholds, apnea, increased work of breathing (retractions, dyspnea, nasal flaring, grunting), PaO₂/FiO₂ ratio < 250, multilobar infiltrates, altered mental status, hypotension, pleural effusion, comorbidities, or unexplained metabolic acidosis.
-
3)
Severe pneumonia was defined by the presence of ≥ major criterion or ≥ 2 minor criteria.
Patients were excluded if they had (1) insufficient clinical data or (2) negative result from virological testing. Approval for this study was obtained from the medical ethics committee of Shenzhen Children’s Hospital, and informed consent forms were signed by the guardians of the hospitalized children.
2.2 Research Methods
2.2.1 Data Collection and Grouping
Utilizing the electronic medical record management system at Shenzhen Children’s Hospital, medical records of children admitted to the PICU with SVP between January 2017 and December 2023 were extracted. Demographic and clinical data, including sex, age, epidemiological features, comorbidities, confirmed pathogens, complications, ancillary diagnostic findings, and therapeutic interventions, were analyzed. Patients were subsequently stratified into survivor and non-survivor groups based on clinical outcomes.
2.2.2 Viral Pathogen Detection
Viral tests were only included if samples were obtained between 72 h before and 48 h after PICU admission, to reflect the likely presence of viruses at the time of PICU admission [5–6]. During hospitalization, respiratory specimens such as tracheal aspirates and bronchoalveolar lavage fluid were collected from the pediatric patients to detect pathogens in the lower respiratory tract. In cases where lower respiratory tract specimens were not obtainable, nasopharyngeal swabs collected during the acute phase of illness were used for virus testing. Each identified virus was recorded individually, even if multiple viruses were present in a single sample. Hospital-acquired viral respiratory infections (HAVRI) was defined as a patient whose number of days from admission to symptom onset exceeded the upper range for the incubation period of the identified virus [7–8] (Table 1). This definition permitted multiple instances of HAVRI during a single hospitalisation, provided that the patient had experienced a complete resolution of symptoms attributed to a respiratory viral infection, a recurrence of symptoms compatible with such an infection, and a time interval greater than the aforementioned incubation period. In the event that the subsequent episode was attributable to a distinct viral agent, it was classified as a HAVRI.
Viral detection was performed using three approaches: (1) Multiplex PCR-CE fragment analysis (Haier Shi GeneTechnology Co., Ltd., China) for nine respiratory pathogens: influenza A/B (IVA/IVB), HBoV, human coronaviruses (HCoV), adenovirus (ADV), RSV, human rhinovirus (HRV), human parainfluenza viruses (HPIV), and human metapneumovirus (HMPV); (2) SARS-CoV-2 detection via RT-qPCR (Bojie Medical Technology Co., Ltd., China) with 2019-nCoV-specific primers/probes; (3) Direct immunofluorescence (Diagnostic Hybrids Inc., USA) targeting five conventional respiratory viruses (IVA/IVB, RSV, ADV, HPIV). and (4) In cases involving severe or critically infected patients where conventional diagnostic methods cannot exclude concurrent infections with other pathogens, metagenomic next-generation sequencing (mNGS) was utilized to identify potential co-infections [9].
2.2.3 Other Pathogen Detection
Bacterial, fungal, and atypical pathogens were systematically screened alongside viral etiologies. Detection methods included serum antibody testing (a positive test indicated by a single serum sample with a Mycoplasma pneumoniae antibody titer ≥ 1:160), respiratory specimen culture, mNGS, and multiplex PCR-CE fragment analysis. The aim of clinical assessment also involved distinguishing between colonization and false positives.
2.3 Statistical Analysis
Categorical variables are expressed as frequencies (percentages), and continuous variables as mean ± SD or median (IQR). Intergroup comparisons employed χ² or Fisher’s exact tests. Multivariable logistic regression analysis calculated adjusted odds ratios (aORs) with 95% CIs. Statistical significance was defined as p < 0.05. Analyses were performed using IBM SPSS Statistics 26.0 (IBM Corp.).
3 Results
3.1 General Information
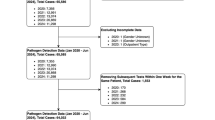
During the observational study period, 7475 pediatric patients were admitted to PICU on a non-elective basis. Of these, 6468 patients(86.5%) who tested negative for other diseases or pathogens were excluded, leaving 1007 patients(13.5%) who met the inclusion criteria. To enhance pathogen detection and accuracy, multiple laboratory methods were employed for testing respiratory specimens from the same patient. Diagnostic testing included multiplex PCR-CE fragment analysis in 608 cases (60.4%), RT-PCR for SARS-CoV-2 detection, direct immunofluorescence in 437 patients (43.4%), and mNGS in 85 patients (8.4%) (Fig. 1).
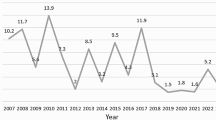
Among these admissions, SVP was diagnosed in 1007 cases (13.5%), with a case fatality rate (CFR) of 6.1% (n = 61). The cohort demonstrated male predominance (63.3% [637/1007]; male-to-female ratio, 1.7:1), with a median pre-PICU illness duration of 3.0 days (IQR 2.0–5.0) and median age of 1.25 years (IQR 0.50–3.83). The cases exhibited an annual distribution as follows: 87cases in 2017, 126 cases in 2018, 206 cases in 2019, 123 cases in 2020, 149 cases in 2021, 100 cases in 2022, and 216 cases in 2023, corresponding to percentages of 8.6%, 12.5%, 20.5%, 12.2%, 14.8%, 9.9% and 21.4%, respectively. Figure 2 illustrates temporal trends in SVP incidence across pre-pandemic, pandemic, and post-pandemic phases of the COVID-19 era.
The distribution by age group was as follows: <1 year (409 patients), 1–3 years (279 patients), 3–5 years (131 patients), 5–10 years (142 patients), and 10–14 years (46 patients), accounting for 40.6%, 27.7%, 13.0%, 14.1%, and 4.6% of patients, respectively. The seasonal distribution revealed 295 cases from December to February, 214 from September to November, 275 from June to August, and 223 from March to May, with percentages of 29.3%, 21.3%, 27.3%, and 22.1%, respectively. The epidemiological distribution of the ten respiratory viruses, classified according to age and season, is presented below (Table 2). Figure 2 delineates 7-year (2017–2023) temporal trends in monthly SVP case volumes and mortality rates within the PICU, stratified across pre-pandemic, pandemic, and post-pandemic epidemiological phases.
The cohort comprised pediatric patients from Guangdong Province, China (n = 1007), predominantly Shenzhen residents (80.2%, n = 808) versus non-local residents (19.8%, n = 199). Healthcare transitions included 29.8% (n = 300) intra-hospital transfers within Shenzhen Children’s Hospital and 10.2% (n = 103) inter-hospital transfers across Guangdong. Community-acquired infections predominated (n = 944, 93.7%) over hospital-acquired infections (n = 63, 6.3%). Among hospital-acquired cases, 71.4% (n = 45) originated from transferred ward patients, while 15.9% (n = 10) involved hospitalization-acquired transmission from symptomatic caregivers.
Among all patients, 92 cases (9.1%) required multiple PICU admissions for SVP, including 19 pre-pandemic, 38 during the pandemic, and 35 post-pandemic, with a median interval of 6.4 months (IQR 2.5–11.3) between admissions. Of these, 56 cases experienced two episodes, and 36 cases had three or more episodes. Twenty patients were readmitted for recurrent infection with the same virus. Comorbidities were present in 78 cases (84.8%), with the top five being moderate-to-severe underweight (36 cases), epilepsy (25 cases), bronchopulmonary dysplasia (10 cases), congenital metabolic disorders (8 cases), and spinal muscular atrophy, congenital heart disease, or immunocompromised status (7 cases each; including acute leukemia, PIDs, and post-transplant biliary atresia).
Notably, 289 patients (28.7%, 289/1007) had a confirmed history of exposure to individuals with respiratory infections, predominantly family members (87.5%, 253/289), before the onset of disease.
3.2 Distribution of Pathogens
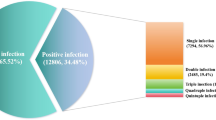
Viral coinfection patterns revealed 86.7% (873/1007) monoinfections, 12.1% (122) dual infections, and 1.2% (12) triple infections. Pathogen distribution demonstrated RSV predominance (28.5%, 287), followed by influenza viruses (26.6%; IVA 20.2% [203], IVB 7.2% [72]), HRV (20.1%, 202), ADV (11.9%, 120), HPIV (8.7%, 88), HBoV (7.8%, 79), HMPV (4.7%, 47), SARS-CoV-2 (3.9%, 39), and HCoV (1.5%, 15).
Age-stratified virome analysis revealed distinct predominant viruses: 1) ≤ 1 year: RSV, HRV, IVA, HPIV; 2) 3–5 years: HRV, RSV, IVA, ADV; 3) 5–10 years: IVA, HRV, ADV, RSV; 4) 10–14 years: HRV, IVA, SARS-CoV-2, RSV (Table 2). Pandemic-phase comparisons demonstrated temporal shifts: (1) Pre-pandemic: IVA, RSV, ADV, IVB (Fig. 3); (2) Pandemic: HRV, RSV, HPIV, HBoV (Fig. 4); (3) Post-pandemic: HRV, RSV, HBoV, IVA (Fig. 5), ordered by chronological detection frequency. Community-acquired SVP cases were predominantly associated with RSV, IVA, HRV, and ADV. In contrast, hospital-acquired infections demonstrated distinct viral profiles, with HPIV, IVA, RSV, and HRV constituting the most frequent pathogens. These four agents– HRV, RSV, HPIV, and IVA– emerged as the principal etiological drivers of SVP, exhibiting significant correlations with recurrent hospitalizations (Table 2).
3.3 Coinfection with Other Pathogens
Among the cohort, 32.6% (n = 328) presented with co-infections: (1) Bacterial: 21.2% (n = 213), primarily Streptococcus pneumoniae (n = 83), Haemophilus influenzae (n = 63), and Staphylococcus aureus (n = 24); (2) Atypical: 8.9% (n = 90), predominantly Mycoplasma pneumoniae (n = 71) and Bordetella pertussis (n = 13); (3) Fungal: 2.5% (n = 25), chiefly Pneumocystis jirovecii (n = 12) and Aspergillus spp. (n = 7).
3.4 Underlying Health Conditions
The cohort exhibited a 53.0% (534/1007) prevalence of comorbidities: (1) Respiratory disorders (17.0%, n = 171), including airway/lung malformations (n = 67) (e.g., pulmonary agenesis, laryngotracheomalacia, and stenosis), bronchopulmonary dysplasia (n = 73), and other conditions (n = 40) (e.g., scoliosis, pectus excavatum, bronchiectasis, pulmonary hemosiderosis, obstructive bronchitis, and congenital pulmonary cystic adenomatoid malformation); (2) Neurological conditions (11.3%, n = 114), including epilepsy (n = 99) and cerebral palsy (n = 32); (3) Cardiovascular diseases (9.4%, n = 95), including congenital heart defects (n = 87), chronic heart failure (n = 32) and cardiomyopathy (n = 7); (4) Hematologic malignancies (6.1%, n = 61), comprising acute leukemia (n = 28), other malignancies (n = 24), and severe thalassemia/severe aplastic anemia treated with hematopoietic stem cell transplantation (n = 9); (5) Congenital metabolic disorders: 2.1% (21), including congenital adrenal insufficiency, glycogen storage diseases, β-galactosidase deficiency, and β-ketothiolase deficiency; (6) Congenital muscle diseases: 2.9% (29)– spinal muscular atrophy (n = 24), fatal hyperosmolar myopathy (n = 5); (7) Primary immunodeficiencies: 1.4% (14); (8) Other conditions: nephrotic syndrome (n = 11), and post-transplant biliary atresia (n = 9); and (9) Nutritional status: 23.2% (234) exhibited moderate-to-severe underweight (Table 3).
3.5 Complications
Acute respiratory failure occurred in 83.9% (845/1007) of patients, stratified as type I (n = 260) and type II (n = 585). ARDS was observed in 59 patients (5.9%) and 53 patients(5.3%) presented with plastic bronchitis. Complications included 8.5% cases of sepsis, 8.1% cases of infection-related encephalopathy (e.g., acute necrotizing encephalopathy in 6 cases, and Influenza-associated encephalopathy in 33 cases), and 2.9% cases of MODS. In addition, Eighteen cases (1.8%) of rhabdomyolysis were also reported (Table 3).
3.6 Treatment and Outcome
All patients received oxygen therapy with the following respiratory support modalities: Noninvasive ventilation (19.5%, n = 196), high-flow nasal cannula (13.8%, n = 139) and invasive mechanical ventilation (35.3%, n = 355). Extracorporeal membrane oxygenation (ECMO) support was required in 11 cases (1.1%), while 33 patients (3.3%) underwent continuous renal replacement therapy (CRRT). Bronchoalveolar lavage was performed under bronchoscopic guidance in 475 cases (47.2%). Systemic corticosteroid therapy with intravenous methylprednisolone was administered to 596 patients (59.2%), and intravenous immunoglobulin (IVIG) therapy was utilized in 506 cases (50.2%).
Following therapeutic intervention, 946 patients (93.9%) achieved clinical improvement and were transferred to general wards. The overall mortality rate was 6.1% (n = 61), with 17 deaths attributed to progression of pre-existing comorbidities, and 16 cases resulting from sepsis-induced MODS. Additional fatal complications included ARDS in 15 patients, infection-associated encephalopathy in 11 cases, and fulminant myocarditis in 2 individuals. The median duration of PICU hospitalization was 4.0 days (IQR 2.0–8.0).
3.7 Stratified Analysis of Death Factors in the Death Group
Among 61 pediatric fatalities attributed to SVP, cases were stratified into purely viral infections (n = 40) and co-infections (n = 21) groups based on pathogen identification. Significant intergroup differences emerged in clinical characteristics: the co-infections cohort demonstrated higher prevalence of age < 3 years (76.2% vs. 42.5%, p = 0.012), primary immunodeficiency diseases (19.0% vs. 0, p = 0.011), sepsis (66.7% vs. 32.5%, p = 0.011), IVIG therapy (85.7% vs. 60.0%, p = 0.039), and bronchoscopy-guided bronchoalveolar lavage utilization (66.7% vs. 22.5%, p = 0.001) (Table 4).
Multivariable logistic regression analysis revealed that age < 3 years (aOR 4.379, 95% CI 1.155–16.603) and sepsis (aOR 4.051, 95% CI 1.111–14.765) remained independently associated with increased mortality risk in children with SVP complicated by coinfections, following adjustment for potential confounders (Table 5).
3.8 Analysis of Risk Factors for Death
Comparative analysis revealed significant disparities between non-survivor and survivor cohorts. The non-survivor group exhibited higher prevalence of prolonged hospitalization (> 9 days; 37.7% vs. 22.0%), moderate-severe underweight (37.7% vs. 22.3%), acute leukemia (11.8% vs. 2.2%), other malignancies (13.1% vs. 1.7%), PIDs (6.6% vs. 1.1%), ARDS (34.4% vs. 4.0%), rhabdomyolysis (18.0% vs. 0.7%), infection-associated encephalopathy (27.9% vs. 6.9%), sepsis (44.3% vs. 6.2%), and MODS (36.1% vs. 0.6%). Conversely, survivors demonstrated higher rates of younger age (< 5 years; 96.6% vs. 90.2%, p = 0.027), shorter PICU stays (2–5 days; 46.0% vs. 31.1%, p = 0.006), and acute respiratory failure (85.2% vs. 63.9%, p<0.001) (Table 3).
Multivariable logistic regression adjusted for disease severity scores identified seven independent mortality predictors: acute leukemia (aOR 6.285, 95%CI 1.959–20.164), other malignant tumors (aOR 15.358, 4.873–48.404), PIDs (aOR 9.204, 2.284–37.095), moderate-to-severe underweight (aOR 3.775, 1.879–7.583), rhabdomyolysis (aOR 5.503, 1.105–27.410), ARDS (aOR 7.787, 3.240-18.715), infection-related encephalopathy (aOR 3.489, 1.306–9.323) and MODS (aOR 24.255, 7.107–82.781) (Table 6).
3.9 Construction of a Predictive Model for Mortality in Patients with SVP
The predictive model demonstrated excellent discriminative capacity for mortality risk in SVP, with a receiver operating characteristic (ROC) curve analysis revealing an area under the curve (AUC) of 0.888 (95% confidence interval [CI]: 0.838–0.938). The model achieved 73.8% sensitivity and 90.2% specificity at the optimal cutoff threshold (Fig. 6). Statistical analyses were performed using IBM SPSS Statistics (version 26.0).
4 Discussion
Lower respiratory infections, particularly pneumonia, are a leading infectious agent of mortality worldwide, claiming over 2 million lives annually, with 672,000 of these being children under five years of age [1, 10]. Among the various pathogens causing pneumonia in children, viruses are the predominant culprits [11]. In a comprehensive study spanning several Asian and African nations, it was revealed that viruses accounted for 61.4% of hospitalizations without HIV infection due to severe pneumonia acquired in the community among children under five years old [12]. In pre-pandemic period, datefrom a multi-center retrospective analysis in the United States show that among hospitalized children withcommunity-acquired pneumonia, 66% of the patients tested positive for respiratory viruses, and the most commonly detected pathogens were RSV (28%), HRV (27%), HMPV (13%), ADV (11%), HPIV (7%), IVA/B (7%), HCoV (5%) [11], Which a little different from our observations, IV, RSV, ADV, HRV were the most predominan viral pathogens. Variations in climate, population distribution characteristics and other factors often lead to differences in the prevalence of viral pathogens in different regions [13–14]. RSV and HRV is still the most predominant virus for the baby and young children [15–16], which is the same as our study.
Changes in the pathogen spectrum have an impact on the effective formulation of public health policies and strategies for controlling infectious diseases. The establishment of a respiratory virus surveillance network and the monitoring of viral changes are conducive to the timely adjustment of the allocation and use of medical resources, ensuring effective prevention and treatment of diseases caused by different pathogens [17]. Furthermore, this allows for the rapid and accurate diagnosis of new respiratory viruses, the effective adjustment of detection strategies and epidemiological surveillance means, and a better grasp of the dynamic changes of the epidemic. Before the COVID-19 pandemic, children with acute viral lower respiratory tract infections exhibited a seasonal peak pattern for IV, RSV and ADV, for the previous three, the epidemic is predominantly in the winter [17–18]; With a notable concentration of adenovirus outbreaks occurring in 2019 prior to the onset of the COVID-19 pandemic, and some similar reports have been made in the past [19]. However, the epidemic peaks of respiratory viruses differ between northern and southern regions of China, such as the areas around Shenzhen in the southern regions, with infection peaking in spring and winter or during the wet rainy season [18, 20]. The prevalence of HRV was consistently high throughout the year and the difference between months was not significant [11, 15, 17, 20], and may be associated with the absence of the envelope of HRV itself, its narrow diameter, its considerable genetic diversity (exceeding 160 serotypes) and its epidemiological inverse correlation with IV(competitive viral inhibition or interference) [21, 22, 23].
Following the onset of the COVID-19 pandemic in December 2019, a marked decline was observed in influenza-associated and adenovirus-associated pneumonia cases, contrasting with significant increases in detection rates of HRV, HPIV, bocavirus HBoV, HCOV, and HMPV, with HRV infections demonstrating the most pronounced epidemiological surge during the pandemic period. A study from South Africa showed a 48% decline in RSV-associated lower lower respiratory infections hospitalisation rates and a 95% decline for influenza in 2020 compared with the pre-pandemic period average [24]. It was mainly attributed to the many non-pharmaceutical interventions (NPIs) implemented to reduce SARS-CoV-2 transmission [17, 24–25]. Despite the challenges of stopping the spread of respiratory viruses in infants and young children due to their unique characteristics, development, compliance issues and limited awareness of autonomous health protection, NPIs have been shown to slow the transmission of COVID-19 in the population and influence the prevalence and spread of other common respiratory viruses [26]. Second, changes in the host’s innate antiviral immune response and competitive viral inhibition or interference may be associated with this variation [21, 22, 23]. As some individuals lack natural immunity and have not previously encountered specific viruses, with relaxation of NPIs, the resulting immunity gap in such settings may have contributed to increased susceptibility and more severe disease when respiratory viruses re-enter the circulation [27]. Our study found a significant increase in the number of RSV, influenza virus and ADV infections in the post-pandemic compared with the pandemic period. Studies also reported that seasonality and burden of IV and RSV infections in 2022 were similar to pre-pandemic levels [24, 28]. In addition, from July 2023 to the present (October 2024), children in China are experiencing a storm of macrolide-resistant Mycoplasma pneumoniae ravaging the country [29].
HAVRI, regardless of virus type, contribute significantly to morbidity and mortality, particularly in patients with underlying malignancy or in the transplant population, and are associated with adverse neonatal outcomes, including prolonged hospital stay, need for ventilatory support, and increased risk of developing bronchopulmonary dysplasia [7, 30]. Pediatric respiratory viruses vary in their clinical presentation but are mainly transmitted by respiratory droplets and contact. Within this cohort, 6.3% of patients acquired the infection nosocomially, 71.4% originated from transferred ward patients, while 15.9% involved hospitalization-acquired transmission from symptomatic caregivers. The infectivity of respiratory viruses in clinical settings remains poorly understood, posing challenges for infection control, especially concerning HAVRI. In line with previous studies [30–31], the predominant viruses causing HAVRI were RSV, IVA, and HPIV, and their epidemiology closely reflects that of the community. These highly transmissible viruses, known for their elevated susceptibility rates, are among the most prevalent RNA viruses affecting children. To prevent cross-infections during the initial phases of hospitalization, comprehensive clinical assessments should be conducted upon transfer within the hospital. This is particularly important during outbreaks of respiratory infections, when the risk of hospital-acquired infections increases in densely populated areas or in inadequately ventilated wards and examination rooms. HAVRI can cause recurrent or worsened pneumonia in children, highlighting the importance of vigilance by healthcare providers. In adults, viral respiratory infections have been implicated in up to a third of ICU admissions with severe pneumonia, with mortality rates comparable to those of confirmed bacterial pneumonia [32].
Although the viral spectrum of respiratory viruses that cause SVP in children has changed, there is no significant change in the pneumonia mortality rate compared to pre-pandemic period. Viral pathogens are often found in children requiring intensive care with respiratory infections, but do not alter all-cause mortality in the selected group studied because the likelihood of viral testing depended on disease and disease severity, and the main analyses were performed only on tested patients [33]. Underlying health conditions are closely related to the severity of pneumonia in children. Within this cohort, 53.0% of the children in this cohort had comorbidities, and diseases of the respiratory system, the cardiovascular system and the nervous system account for 17%, 14.04% and 11.3% respectively. Comorbidities greatly increase the risk of serious outcomes associated with common viral infections, especially cardiovascular and pulmonary disease [4, 33–34]. Additionally, a 12-year retrospective study highlighted severe acute malnutrition and moderate-to-severe underweight as risk factors for pediatric pneumonia-related mortality [35], and 23.2% of children in our study were reported to have moderate-to-severe underweight. Within this patient cohor, acute leukemia, other malignant tumors, and primary immunodeficiency diseases, were identified as significant factors elevating the risk of mortality and also at high risk of developing hospital acquired infections. Similar to previous reports [7, 31, 34, 36–37], our study found that children with these conditions, especially those under 3 years of age (aOR 4.379, 95%CI 1.155–16.603), are more likely to develop severe secondary infections, sepsis, and multi-organ and multisystem involvement, which increases the risk of complications and mortality from viral infections. Mechanistically, insufficient airway epithelial anti-spike secretory IgA (S-IgA) in young children compromises viral clearance through reduced RNA load neutralization and diminished infectivity control [38–39]. Anatomically narrower airways and reduced ciliary motility in this age group promote secretion retention, fostering pathogen proliferation. Furthermore, underdeveloped swallowing coordination and elevated aspiration risk predispose to secondary bacterial colonization.
The dissemination of information regarding the underlying diseases and serious complications associated with childhood illnesses can facilitate a more comprehensive understanding of the health status of children among parents and caregivers. Regular medical check-ups can assist in the early detection of underlying diseases, while prompt consultation at the onset of disease can help to avert complications. Vaccination is the most cost-effective and efficient means of preventing SVP and its serious complications and reducing the spread of respiratory viruses [40]. It is regrettable that, with the exception of IV and SARS-CoV-2, there are no vaccines that have been specifically approved for use against the majority of respiratory viruses. Nevertheless, vaccination against other respiratory pathogens can also assist in the prevention of viral pneumonia. A study conducted in South Africa demonstrated that the pneumococcal conjugate vaccine was effective in preventing one-third of viral pneumonia cases, which was likely due to its ability to prevent superimposed bacterial co-infections [41].
The respiratory virus can cause organ damage post-infection either through direct invasion of tissue cells leading to pathogenic consequences or indirectly by releasing cytokines and triggering cell or antibody-mediated immune responses [42–43]. The occurrence of “cytokine storms” has been associated with virus-associated encephalopathy and ARDS [44]. Apart from influenza viruses, infections caused by various respiratory viruses can also result in central nervous system consequences such as meningitis and encephalopathy, particularly in young individuals [43, 45]. In our study, 8.1% of children with SVP developed infection-associated encephalopathy, and the proportion was significantly higher in the group that died. Regrettably, due to limitations of the retrospective study, we were unable to ascertain the levels of inflammatory markers in the bronchoalveolar lavage fluid and cerebrospinal fluid of deceased children with simple viral infections and co-infections.
This study has several methodological limitations requiring cautious interpretation. First, the single-center retrospective design inherently constrains sample size adequacy and generalizability, necessitating validation through prospective multi-center cohorts. Second, respiratory specimen collection within 48–72 h post-PICU admission (without standardized collection window) introduces potential diagnostic bias due to variable viral shedding kinetics across pathogens. Third, the absence of viral genotypic characterization precludes delineation of strain-specific virulence or epidemiological patterns. Furthermore, evolving diagnostic modalities warrant consideration: the progressive adoption of multiplex PCR and mNGS over the 7-year study period enhanced pathogen detection sensitivity but concurrently increased risks of misinterpreting respiratory commensals as pathogens. This study employed mNGS for respiratory pathogen detection in 85 patients(8.4%) with specific clinical indications. This targeted application mitigates potential false-positive rates through avoidance of indiscriminate testing in low-prevalence populations, thereby may reducing diagnostic ambiguity associated with non-selective mNGS utilization. Additionally, reliance on viral antigen detection assays with suboptimal sensitivity during 2017–2018 surveillance phases may have introduced diagnostic ascertainment bias.
Conclusion.
In summary, the impact of the COVID-19 pandemic has led to significant changes in the viral spectrum among critically ill children with viral pneumonia in the PICU. Among critically ill children with viral pneumonia in the PICU, independent risk factors associated with mortality include acute leukemia, other malignancies, primary immunodeficiency, moderate-to-severe underweight, rhabdomyolysis, ARDS, infectious related encephalopathy, MODS. These factors can be utilized to develop a reliable risk prediction model with strong predictive value. Early identification of underlying conditions, prompt treatment, and tailored management strategies based on these criteria contribute to reducing mortality rates in SVP.
Data Availability
No datasets were generated or analysed during the current study.
References
Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet 2020; 396(10258): 1204-22.
He C, Liu L, Chu Y, et al. National and subnational all-cause and cause-specific child mortality in China, 1996–2015: a systematic analysis with implications for the sustainable development goals. Lancet Glob Health. 2017;5(2):e186–97.
Li M, Wang H, Tian L et al. COVID-19 vaccine development: milestones, lessons and prospects.
Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the pediatric infectious diseases society and the infectious diseases society of America. Clin Infect Dis. 2011;53(7):e25–76.
Katie M, Andrew B, Nelson A et al. Impact of viral respiratory pathogens on outcomes after pediatric cardiac surgery. Pediatr Crit Care Me 2017; 18(3).
Justin L, Nicholas GR, Ron B, Trish MP, Kenrad EN. Derek A T C. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis 2009; 9(5).
Chow EJ, Mermel LA. Hospital-Acquired respiratory viral infections: incidence, morbidity, and mortality in pediatric and adult patients. Open Forum Infect Di. 2017;4(1):ofx006.
Andreas C, Olli K, Varpu E et al. Human bocaviruses and paediatric infections. Lancet Child Adolesc 2019; 3(6).
Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20(6):341–55.
Kyu HH, Vongpradith A, Sirota SB, et al. Age–sex differences in the global burden of lower respiratory infections and risk factors, 1990–2019: results from the Global Burden of Disease Study 2019. The Lancet Infectious Diseases 2022; 22(11): 1626-47.
Jain S, Williams DJ, Arnold SR et al. Community-Acquired pneumonia requiring hospitalization among U.S. Children. New Engl J Med 372(9): 835–45.
O’Brien KL, Baggett HC, Brooks WA, et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019;394(10200):757–79.
Li Z, Zhang H, Ren L, et al. Etiological and epidemiological features of acute respiratory infections in China. Nat Commun. 2021;12(1):5026.
Baker RE, Mahmud AS, Wagner CE, et al. Epidemic dynamics of respiratory syncytial virus in current and future climates. Nat Commun. 2019;10(1):5512.
Lei C, Yang L, Lou CT, et al. Viral etiology and epidemiology of pediatric patients hospitalized for acute respiratory tract infections in Macao: a retrospective study from 2014 to 2017. Bmc Infect Dis. 2021;21(1):306.
Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med. 2012;166(8):700–6.
Perez A, Lively JY, Curns A, et al. Respiratory virus surveillance among children with acute respiratory Illnesses - New vaccine surveillance network, united States, 2016–2021. Mmwr-Morbid Mortal W. 2022;71(40):1253–9.
Li ZJ, Zhang HY, Ren LL, et al. Etiological and epidemiological features of acute respiratory infections in China. Nat Commun. 2021;12(1):5026.
Duan YL, Zhu Y, Xu BP, et al. [Multicenter study of human adenovirus infection in pediatric community-acquired pneumonia in China]. Zhonghua Er Ke Za Zhi. 2019;57(1):27–32.
Wang H, Zheng Y, Deng J, et al. Prevalence of respiratory viruses among children hospitalized from respiratory infections in Shenzhen, China. Virol J. 2016;13(1):39.
Wu A, Mihaylova VT, Landry ML, Foxman EF. Interference between rhinovirus and influenza A virus: a clinical data analysis and experimental infection study. Lancet Microbe. 2020;1(6):e254–62.
Nickbakhsh S, Mair C, Matthews L, et al. Virus-virus interactions impact the population dynamics of influenza and the common cold. P Natl Acad Sci Usa. 2019;116(52):27142–50.
Pinky L, Dobrovolny HM. Coinfections of the respiratory tract: viral competition for resources. PLoS ONE. 2016;11(5):e0155589.
Izu A, Nunes MC, Solomon F, et al. All-cause and pathogen-specific lower respiratory tract infection hospital admissions in children younger than 5 years during the COVID-19 pandemic (2020–22) compared with the pre-pandemic period (2015–19) in South Africa: an observational study. The Lancet Infectious Diseases 2023; 23(9): 1031-41.
Li Y, Wang X. Unveiling the viral aetiologies of lower respiratory infections. Lancet Infect Dis. 2024;24(9):938–9.
Armero G, Guitart C, Soler-Garcia A, et al. Non-Pharmacological interventions during SARS-CoV-2 pandemic: effects on pediatric viral respiratory infections. Arch Bronconeumol. 2024;60(10):612–8.
Cohen R, Ashman M, Taha M, et al. Pediatric infectious disease group (GPIP) position paper on the immune debt of the COVID-19 pandemic in childhood, how can we fill the immunity Gap?? Infect Dis now. 2021;51(5):418–23.
Tang Y, Dang X, Lv M, et al. Changes in the prevalence of respiratory pathogens in children due to the COVID-19 pandemic: A systematic review and meta-analysis. J Infect. 2023;86(2):154–225.
Li H, Li S, Yang H, Chen Z, Zhou Z. Resurgence of < em > mycoplasma pneumonia by macrolide-resistant epidemic clones in China. Lancet Microbe. 2024;5(6):e515.
Manchal N, Mohamed MRS, Ting M, et al. Hospital acquired viral respiratory tract infections: an underrecognized nosocomial infection. Infect Disease Health. 2020;25(3):175–80.
Nichols WG, Guthrie KA, Corey L, Boeckh M. {Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy}. Clin Infect Dis. 2004;39(9):1300–6.
Choi SH, Hong SB, Ko GB, et al. Viral infection in patients with severe pneumonia requiring intensive care unit admission. Am J Resp Crit Care. 2012;186(4):325–32.
Moynihan KM, McGarvey T, Barlow A, et al. Testing for common respiratory viruses in children admitted to pediatric intensive care: epidemiology and outcomes. Pediatr Crit Care Me. 2020;21(6):e333–41.
Thorburn K. Pre-existing disease is associated with a significantly higher risk of death in severe respiratory syncytial virus infection. Arch Dis Child. 2009;94(2):99–103.
Lazzerini M, Seward N, Lufesi N, et al. Mortality and its risk factors in Malawian children admitted to hospital with clinical pneumonia, 2001-12: a retrospective observational study. Lancet Global Health. 2016;4(1):e57–68.
Michael CS, Jason WC, Melania MB, Devon OA, Xiaoyan S, Susanna S. A multicenter outcomes analysis of children with severe viral respiratory infection due to human metapneumovirus. Pediatr Crit Care Me 2013; 14(3).
Robert C, Sr W, Paul AC, Jay HB, Ancilla WF, Parthiv JM, Caroline BH. Fatality rates in published reports of RSV hospitalizations among high-risk and otherwise healthy children. Curr Med Res Opin 2010; 26(9).
Miyamoto S, Nishiyama T, Ueno A, et al. Infectious virus shedding duration reflects secretory IgA antibody response latency after SARS-CoV-2 infection. P Natl Acad Sci Usa. 2023;120(52):e1980159176.
Donald K, Petersen C, Turvey SE, Finlay BB, Azad MB. Secretory IgA: linking microbes, maternal health, and infant health through human milk. Cell Host Microbe. 2022;30(5):650–9.
Yang Z, Feng T, Guan W, et al. Chinese expert consensus on immunoprophylaxis of common respiratory pathogens in children (2021 edition). J Thorac Dis. 2022;14(3):749–68.
Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10(8):811–3.
Gupta N, Richter R, Robert S, Kong M. Viral sepsis in children. Front Pediatr. 2018;6:252.
Gu-Lung L, Joseph PM, Simon BD, Andrew JP. Epidemiology and immune pathogenesis of viral sepsis. Front Immunol 2018; 9(0).
Nicola C, Sreya G, Maria DS et al. Viral respiratory pathogens and lung injury. Clin Microbiol Rev 2021; 34(3).
Gemma LS, Catherine LK, Lucy D et al. Respiratory syncytial Virus-Associated neurologic complications in children: A systematic review and aggregated case series. J Pediatr-Us 2021; 239(0).
Funding
This work was supported, in part, by the Guangdong Natural Science Foundation (2021A1515010133), Guangdong High-level Hospital Construction Fund, 2025 Joint Funding Projects of Universities and Enterprises (SL2024A03J00547), Guangzhou Science and Technology Plan (Dengfeng Hospital) Municipal Key Laboratory Construction Project (2023A03J0209), and Sanming Project of Medicine in ShenZhen(SZSM202211034). The sponsors were not involved in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Huabao Chen, Lidan Zhang, and Xing Nie designed the study and participated in the drafting of the manuscript. Li Wang participated in data analysis and drafting of the manuscript. Liangliang Kang and Yucong Zhang participated in the data collection. Zhuanggui Chen participated in supervision of this study. Yating Li and Yuhui Wu designed the study, drafted the manuscript, and participated in the critical revisions of the draft. All authors contributed toward data analysis, drafting, and critically revising the paper and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was conducted in compliance with the protocol approved by the Ethics Committee of the Shenzhen Children’s Hospital ( Research approval number: 2022033).
Conflict of interest
The authors report no conflicts of interest associated with this work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, H., Zhang, L., Nie, X. et al. Epidemiology and Mortality Risk of Severe Viral Pneumonia During the Pre-Pandemic, COVID-19 Pandemic and Post-Pandemic Era: A Retrospective Study of Hospitalized Children in ShenZhen, China Between 2017 and 2023. J Epidemiol Glob Health 15, 53 (2025). https://doi.org/10.1007/s44197-025-00398-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44197-025-00398-7