Abstract
Observational studies suggest a reduction in fatal or severe COVID-19 disease with the use of ACE2 inhibitors and statins. We implemented a randomized controlled tree-arm open label trial evaluating the benefits of adding telmisartan (TLM) or atorvastatin (ATV) to lopinavir boosted ritonavir (LPVr) on the SARS-CoV-2 nasopharyngeal viral load in patients with mild / moderate COVID-19 infection in Côte d’Ivoire. RT-PCR positive COVID-19 patients ≥ 18 years, with general or respiratory symptoms for less than 7 days were randomized (1:1:1) to receive LPVr (400 mg/100 mg twice daily), LPVr + TLM (10 mg once daily) or LPVr + ATV (20 mg once daily) for 10 days. The primary endpoint was viro-inflammatory success defined as a composite variable at day 11: Ct ≥ 40 and C-reactive protein < 27 mg/L. We randomized 294 patients: 96 to LPVr, 100 to LPVr + TLM, 98 to LPVr + ATV arms. Baseline characteristics were well balanced between arms. In the primary analysis (missing = failure), 46% patients in the LPVr arm reached viro-inflammatory success at day 11 vs 43% in the LPVr + TLM arm (p = 0.69) and 43% in the LPVr + ATV arm (p = 0.68). The median time from baseline to resolution of COVID-19 related symptoms was not different between arms. Nine patients were hospitalized: 2 in the LPVr arm, 5 in the LPVr + TLM arm and 2 in the LPVr + ATV arm and 4 patients died. Among adults with mild to moderate COVID-19 infection, the addition of telmisartan or atorvastatin, to the standard LPVr treatment is not associated with a better virological or clinical outcome.
Trial registration: NCT04466241, registered on 10/07/2020
Similar content being viewed by others
Introduction
Very soon after the beginning of the COVID-19 pandemic, many repurposed drugs were proposed to treat SARS-CoV-2, based on their pharmacochemical properties. Among them, lopinavir boosted by ritonavir (LPVr) showed initial promising clinical results due to its capacity to inhibit SARS-CoV2 replication in vitro, and was initially used in monotherapy as an empirical antiviral treatment in numerous countries before being not recommended due to futility, toxicity and potential drug-drug interaction because of CYP450 ritonavir inhibition1,2,3. Thus, LPVr was the standard treatment recommended for COVID-19 in Côte d’Ivoire in end 2020 and 2021.
Among other promising drugs, statins and ACE2 inhibitors were rapidly identified because of their antiviral and anti-inflammatory properties and because they cause interference with ACE2 signaling, the target of SARS-CoV-2 in vivo4,5,6. Both drugs are also affordable and widely available.
Thus, a multitude of observational studies and meta-analyses of these studies suggested a reduction in fatal or severe COVID-19 disease with the use of statins7,8,9,10,11,12. Other studies showed equivocal results suggesting the need for randomized control trials13,14.
The same pattern was observed for ACE2 inhibitors, with many observational studies showing discordant results15,16,17,18,19 and the need for well-designed randomized controlled studies.
It is remarkable that combination strategies were poorly evaluated during the COVID-19 pandemic, whereas these strategies have been successful during previous viral infection epidemics (HIV, Ebola Virus Disease), particularly in the early stages in those with high virus load and replication capacity20,21.
A reduction of the community viral load is also a cornerstone of the prevention of transmission22. It requires the antiviral to be affordable, well tolerated, oral, easy to manage and prescribed early in the course of infection to provide individual and collective benefits, like other test-and-treat strategies that have proved efficient in other contexts23.
We therefore conducted a randomized controlled trial to evaluate the benefits of adding statins or telmisartan to LPVr on the SARS-CoV-2 nasopharyngeal viral load in patients with mild to moderate COVID-19 infection in Côte d’Ivoire.
Methods
Study design
The INTENSE-COV study was a phase II superiority multicenter prospective randomized controlled trial in 3 open-label parallel arms (ratio 1:1:1), assessing the effect of telmisartan or atorvastatin plus LPVr among mild to moderate COVID-19 adults recently infected by SARS-CoV-2.
The trial was conducted at two designated COVID-19 facilities at the Service des Maladies Infectieuses et Tropicales, CHU de Treicheville, and the Centre de Traitement des Maladies Infectieuses, CHU de Yopougon in Abidjan, Ivory Coast.
The trial was approved by the institutional national ethics committee (Comité National d’Ethique de la Vie et de la Santé (CNEVS), July 27, 2020) and was conducted in accordance with the 1964 Helsinki Declaration and the French and Ivorian regulations on clinical trials. The trial sponsor was the ANRS/Maladies Infectieuses Emergentes, Paris France. We obtained written informed consent from all patients. The trial was registered on clinicaltrial.gov (NCT04466241, registered on 10/07/2020) and the protocol is available online.
Patients’ recruitment
All RT-PCR positive COVID-19 patients ≥ 18 years, ambulatory or hospitalized with clinical general or respiratory symptoms for less than 7 days were included in the trial if they signed the informed consent.
The exclusion criteria were severe illness (oxygen > 4 L/min to reach capillary oximetry > 94%), WHO Ordinal Scale for Clinical Improvement (WHO-OSCI) > 5, weight < 35 kg, documented significant liver disease/dysfunction (aspartate or alanine aminotransferase > 3 times the upper range), myopathy, rhabdomyolysis (creatine phosphokinase > 5 times the upper range), cirrhosis, HIV infection with treatment including a ritonavir boosted protease inhibitor, electrocardiogram showing a QTc interval > 500 ms, known allergy or intolerance to statins or telmisartan, use of statin or ARBs or conversion enzyme inhibitor within the last 30 days, use of drugs known to have significant interaction with LPVr or atorvastatin, pregnancy, breastfeeding, inability to sign a consent form.
Demographic information, including age, gender, and residential address was recorded for all recruited patients. All patients were clinically evaluated for comorbid conditions including diabetes mellitus, hypertension, cardiovascular disease, chronic kidney disease and chronic liver disease, drug history, and COVID-19 related symptoms and signs.
Randomization and interventions
The randomization list was computer-generated and stratified by moderate or mild disease COVID-19 severity according to the WHO-OSCI, with fixed block size (9 for the 12 first blocks, then 6). Patients were randomized (1:1:1) to receive LPVr (400 mg/100 mg twice daily), LPVr + TLM (10 mg once daily) or LPVr + ATV (20 mg once daily) for 10 days. All patients received conventional medical therapy according to the COVID-19 center’s treatment protocol and the physician’s clinical judgment. We chose the doses and duration of therapy based on studies on the use of statins in non-COVID pneumonias and on virological studies describing the decrease in the SARS-CoV-2 viral load at day 1024.
Procedures
At day 0, we screened patients for eligibility: the investigators reported their medical history and current treatment, performed clinical and laboratory evaluations and assessed self-reported symptoms related to COVID-19. They prescribed the trial treatment to eligible patients according to the randomization, free of charge.
The investigators performed measurements of vital signs, clinical examinations, recorded medical history and treatments, and assessed self-reported symptoms related to COVID-19 at days 1, 3, 7, 11, 14, 21 and 28. A dedicated research assistant could conduct visits on days 14 and 21 via phone contact if the SARS-CoV-2 viral load was negative at day 11. The safety assessment included daily monitoring for symptoms and adverse events, an electrocardiogram at days 1 and 3, and clinical laboratory measures at days 3, 7, and 11 (plasma cell blood count, creatinine, estimated glomerular filtration rate (CKD-EPI formula) kaliemia, glycemia, transaminases, creatine phosphokinase, gamma glutamyl transferase). Trained research nurses performed SARS-CoV-2 viral RNA detection by RT-PCR assay (COVID-19 genesig® Real-Time PCR Coronavirus, Primerdesign Ltd, Chandler’s Ford, UK), systematically at days 3, 7 and 11, and at days 14, 21 and 28 if the viral load was still positive on the previous sample. The virologists performing SARS-CoV-2 RT-PCR (Ivory Coast Pasteur Institute, Abidjan, Côte d’Ivoire) were unaware of the randomization arm. Patients had to contact investigators in case of adverse events or a worsening of symptoms. Follow-up ended at 28 days. The investigators and clinical research assistants at each site carried out data collection with the secure electronic data capture tool REDCap®. Data management and database validation and cleaning were done by trial staff from the Clinical Trial Unit PAC-CI, Abidjan, Ivory Coast.
Outcomes
The variable cycle threshold (Ct) is the number of cycles in the SARS-CoV-2 RT-PCR, and is inversely related to viral load. The primary endpoint was viro-inflammatory success defined as a composite variable at day 11: Ct ≥ 40 and C-reactive protein (CRP) < 27 mg/L. The Ct threshold was the one commonly used at the Pasteur Institute for viral load negativity. We chose the CRP threshold due to its predictive value for severe COVID-19, shown in a previous study25.
Other outcomes included Ct and CRP kinetics, clinical worsening (WHO-OSCI), complete resolution of COVID-19 related symptoms, adverse events (severity assessed through the Common Terminology Criteria for Adverse Events v5.0, relation of severe or life threatening events to trial treatment assessed by an adjudication committee), mortality and compliance with trial treatment at visits 1, 3, 7 and 11.
Statistical analysis
We estimated that a sample size of 294 patients (98 patients per arm) would provide the trial with 80% power to detect an improvement of 50% (from 40 to 60%) in viro-inflammatory success at day 11 with any of the combination therapies compared to the monotherapy, with a first type error of 2.5% for each test and a loss of follow up of 1%.
We analyzed the primary endpoint on the intention-to-treat population, which considered all patients in their randomization arm. We imputed all missing data by failure in the primary analysis. We performed an adjusted analysis through a logistic regression to assess the effect of COVID-19 infection severity. We also performed a sensitivity analysis on the complete case population, and another one on the complete case population with a Ct threshold of 33, the non-contagious threshold commonly used by the Pasteur Institute.
Moreover, the national recommendations changed during trial progress. The day 11 Ct measure was no longer mandatory for patients who have recovered. Therefore, the investigators stopped prescribing RT-PCR for enrolled patients in such cases, despite the trial protocol, leading to a missing primary endpoint for 41 patients. When the trial team discovered this, it reinstated the systematic day 11 Ct measure in the trial. The DSMB approved the proposal of a post hoc analysis with the day 7 Ct value carried forward to day 11 for the 41 patients, and missing data imputed by failure for the others.
Finally we used the Kaplan–Meier method to estimate the symptom-free probability by arm and logrank test to compare arms.
We performed all statistical analyses with the SAS® software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Role of the funding
The funder of the study (ANRS/MIE) played no role in the study design, data collection, data analysis, interpretation of outcomes, or report writing. All authors have full access to all the data. The first and last authors had final responsibility for the decision to submit for publication.
Results
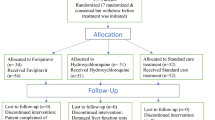
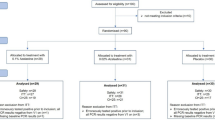
Accrual ran from November 27, 2020 to October 4, 2021. Among the 329 patients with positive SARS-CoV-2 RT-PCR at the two investigator sites, we consecutively enrolled and randomized 294 (Fig. 1): 96 to the LPVr arm, 100 to the LPVr + TLM arm and 98 to the LPVr + ATV arm. Non-inclusion was mainly due to loss of follow up between screening and randomization (28 patients).
The baseline characteristics were well balanced between arms (Table 1). The most frequent COVID-19 related symptoms before the baseline were fatigue (81% patients), cough (61%), anosmia (53%), cephalgia (46%), ageusia (45%) and general malaise (38%) (Fig. 2). Patients had a median duration from the first symptom onset to inclusion of 5 days4,5,6. Most patients received antibiotics (89%), and far fewer anticoagulants (39%).
The kinetics of SARS-CoV-2 RT-PCR results (Ct value) up to day 11 and of CRP up to day 28 were similar in all arms (Fig. 3). A majority of patients in all arms suppressed their viral load (Ct ≥ 40: LPVr 58%, LPVr + TLM 55%, LPVr + ATV 57%) and nearly all had a normal CRP (CRP < 27 mg/L: LPVr 98%, LPVr + TLM 97%, LPVr + ATV 98%) (Table 2). In the primary analysis, 46% patients in the LPVr arm reached viro-inflammatory success at day 11 vs 43% in the LPVr + TLM arm (p = 0.69) and 43% in the LPVr + ATV arm (P = 0.68). In the complete case analysis, viro-inflammatory success was reached at day 11 in 57% patients in the LPVr arm vs 52% in the LPVr + TLM arm (P = 0.56) and 57% in the LPVr + ATV arm (P = 0.96). There was no significant effect of the severity of COVID-19 infection on viro-inflammatory success at day 11 but a slight tendency in the adjusted analysis (P = 0.09). However, the frequency of moderate infection was only 12% such that any such result is questionable. Also, we found no difference between arms in the sensitivity analysis with RT-PCR threshold of 33 (P = 0.59 and 0.85), and in the post hoc analysis day 7 Ct values carried forward to day 11 for the 41 patients and missing data imputed by failure for the others (P = 0.49 and 0.68).)
At day 11, 85% of patients the arm LPVr had ambulatory mild disease without activity limitation, the lowest level of the WHO-OSCI scale, vs 91% in the LPVr + TLM arm (P = 0.40) and 85% in the LPVr + ATV arm (P = 0.79).
The median time from baseline to complete resolution of COVID-19 related symptoms was 10 days with an interquartile range of7,8,9,10,11,12,13,14,15,16,17,18,19 in the LPVr arm vs 14 days9,10,11,12,13,14,15,16,17,18,19,20,21 in the LPVr + TLM arm (logrank test, P = 0.24) and 12 days7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22 in the LPVr + ATV arm (P = 0.23).
No COVID-19 symptom was declared to have worsened during follow up. However, 9 patients were hospitalized: 2 in the LPVr arm, 5 in the LPVr + TLM arm and 2 in the LPVr + ATV arm. Moreover, 4 patients died, without any relation to the trial treatment: 3 in the LPVr arm (digestive hemorrhage, acute respiratory failure, suicide), and 1 in the LPVr + ATV arm (acute respiratory failure). In addition, we observed 3 severe adverse events (diarrhea and rectal hemorrhage related to anticoagulants in 1 patient, hypotension related to TLM in 1 patient) and 5 moderate adverse events (nausea in 2 patients and abdominal pain, myalgia and D-dimers increase, in 1 patient each). There were 283 mild adverse events, mainly diarrhea (21% patients), nausea (11%), vomiting (6%), dyspnea (5%) and coughing (5%). There were more patients with at least one adverse event of any type and severity in the LPVr + ATV arm (66%) than in the LPVr arm (54%, P = 0.09), and the difference was statistically significant when restricted to digestive adverse events of any severity (56% vs 38%, P = 0.01). These rates were also higher in the LPVr + TLM arm than in the LPVr arm but without statistical significance (P = 0.34 for any adverse events, p = 0.18 for digestive adverse events).
Only 1 patient in the LPVr arm discontinued the trial treatment at day 9 instead of day 10. For the visits at days 1, 3, 7 and 11 taken together, the rate of missed intakes over the last 3 days before the visit was 3%, without any difference between arms (P = 0.26 and 0.99).
Discussion
In this randomized controlled trial, we found no impact of telmisartan or atorvastatin in combination with lopinavir boosted by ritonavir on the virological and clinical outcomes in mild or moderate COVID-19.
Despite numerous and large-scale previous observational studies showing a benefit of statins on COVID-19, two recent randomized trials showed no benefit for the clinical outcome (clinical deterioration to WHO-OSCI) in patients treated with statins versus standard care for mild/moderate COVID-1926,27, and two additional randomized study versus placebo showed no benefit in intensive care in terms of the risk of complication or death28,29.
The present study is the first to evaluate a virological endpoint. In it, we have shown that the addition of atorvastatin to LPV/r does not lead to an accelerated decrease in the viral load nor to an improvement in clinical conditions versus LPV/r alone.
Taken together, these four trials argue against a clinical and virological effect of statins on the course of COVID-19 and these statins should no longer be prescribed in this context.
It is unfortunate that the pleiotropic action of statins sparked by in-vitro and in-vivo studies, showing a reduction in inflammation, thrombosis in models of sepsis and pneumonias30,31 and a specific action in the context of COVID-19 including interference with ACE2 signaling6, are not relevant when virological and clinical outcomes are considered. The results reported by many observational studies and meta-analyses showing a positive association between the use of statins and favorable clinical outcomes have probably suffered from heavy indication bias, resulting in a non-comparability of the samples studied.
Moreover, the addition of telmisartan to LPV/r did not improve virological and clinical outcomes in our population. The hypothesis of the involvement of the renin angiotensin system in the inflammatory process triggered by the entry of SARS-CoV-2 into the tissues (lung in first place) considers that the down-regulation of ACE2 causes an imbalance which results in an elevation of tissue concentrations of angiotensin II (pro-inflammatory) and a concomitant reduction in angiotensin 17 (anti-inflammatory). This imbalance could induce the development of AT1 receptor-dependent processes, leading to the release of pro-inflammatory cytokines, in turn triggering a cascade leading, in severe cases, to Acute Respiratory Distress Syndrome (ARDS)32.
The only previous randomized controlled study conducted with the ARB telmisartan published to date suggests that the use of this drug could reduce morbidity and mortality in hospitalized patients infected with SARS-CoV-2 through its anti-inflammatory effects33, as do previous observational studies. Our results with mild/moderate COVID-19 are not in line with these, as we have shown no additional virological effect, no clinical effect and no additional anti-inflammatory effect. The profiles of the patients were not the same, since nearly 70% of patients in Duarte et al. required additional O2; however, the level of systemic inflammation was almost the same, with CRP at 6 and 3 mg/L in both studies at entry. The very high mortality (23%) in the control group in the study by Duarte et al. in patients with quite low inflammation at baseline comes as something of a surprise, as it is far from what we observed in our study in Côte d’Ivoire, with an overall mortality of 1.4%. But recently, The Clarity trial also found in patients admitted to hospital for covid-19, mostly with mild disease and not requiring oxygen, no evidence of benefit for ARB on severity score34
Although there is only few data on viral genomic sequences on Côte d’Ivoire, the second pandemic wave in 2021 (period of the study) was mainly driven by the spread of the Alpha (B.1.1.7) and Eta (B.1.525) variants, but we assume it has not affected our results35. Indeed, unlike direct antiretrovirals or monoclonal antibodies, the presumed mechanism of action of statins and ARBs is anti-inflammatory, antioxidant and associated with modulation of the ACE-2 receptors on the surface of the pulmonary epithelium and do not depend on viral genomic determinants.
Our trial was set up when LPV/r was the standard treatment for COVID-19 in Côte d’Ivoire. In the absence of an alternative, given the satisfactory overall tolerance and despite the demonstration of the absence of impact of LPV/r on the evolution of COVID-19, we kept LPV/r as a standard of care throughout the test.
The main limitations of our study are the lack of blinding and placebo, the exclusion of severe COVID-19 patients on randomization, and the low number of enrolled patients. Another limitation is the restriction to patients showing a relatively short period of time from symptom onset to randomization. The clinical efficacy of the application of an ARB or statin may have been conditioned by the time lapse between the start of the inflammatory process induced by the SARS-CoV-2 and the moment of its administration and the dose of the treatment, which was limited at 10 mg for telmisartan and 20 mg for atorvastatin to prevent side effects. Moreover, since symptom reporting is highly subjective, we cannot rule out the possibility that some patients might have had a marginally longer course of the disease at randomization. However, we believe there are no differences between arms in this respect. The inflammatory syndrome at inclusion was globally lower than expected with a median CRP of 3 mg/l [IQR:1–9]; we cannot rule out an efficacy of stains/ARB in patients with more pronounced inflammation at treatment start. The major flaw in our trial was the rate of missing data in the primary endpoint (20%). This stemmed from logistical difficulties in collecting Ct values from the Pasteur Institute. Consequently, missingness was independent from the randomization arm. Additionally, all analyses led to similar conclusions. Altogether, we are confident that this flaw has not biased our results.
Conclusion
Among adults with mild to moderate COVID-19 infection, the addition of telmisartan or atorvastatin to the standard treatment lopinavir/ritonavir is not associated with a better virological or clinical outcome.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Cao, B. et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 382, 1787–1799. https://doi.org/10.1056/NEJMoa2001282 (2020).
Reis G, dos Santos Moreira Silva EA, Medeiros Silva DC, Thabane L, Singh G, Park JJH, et al. Effect of Early Treatment With Hydroxychloroquine or Lopinavir and Ritonavir on Risk of Hospitalization Among Patients With COVID-19 The TOGETHER Randomized Clinical Trial. JAMA Network Open, https://doi.org/10.1001/jamanetworkopen.2021.6468. (2021).
Ford, N. et al. Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: Initial assessment. JIAS https://doi.org/10.1002/jia2.25489 (2020).
Albini, A., Di Guardo, G., Noonan, D. M. & Lombardo, M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern Emerg. Med. 15, 759–766. https://doi.org/10.1007/s11739-020-02364-6 (2020).
Teixeira, L. et al. Simvastatin downregulates the SARS-CoV-2-induced inflammatory response and impairs viral infection through disruption of lipid rafts. Front. Immunol. https://doi.org/10.3389/fimmu.2022.820131 (2022).
Fedson, D. S., Opal, S. M. & Rordam, O. M. Hiding in plain sight: an approach to treating patients with severe COVID-19 infection. Mbio https://doi.org/10.1128/mBio.00398-20 (2020).
Kow, C. S. & Hasan, S. S. Meta-analysis of effect of statins in patients with COVID-19. Am. J. Cardiol. 134, 153–155. https://doi.org/10.1016/j.amjcard.2020.08.004 (2020).
Shen, L. et al. Statin use and in-hospital mortality in patients with COVID-19 and coronary heart disease. Sci. Rep. https://doi.org/10.1038/s41598-021-02534-2 (2021).
Li, W. et al. Statin use in hospitalized patients with COVID-19: A comprehensive analysis of the New York City public hospital system. Am. J. Med. 135, 897–905. https://doi.org/10.1016/j.amjmed.2022.02.018 (2022).
Pal, R., Banerjee, M., Yadav, U. & Bhattacharjee, S. Statin use and clinical outcomes in patients with COVID-19: An updated systematic review and meta-analysis. Postgrad. Med. J. 98, 354–359. https://doi.org/10.1136/postgradmedj-2020-139172 (2021).
de Mesquita, C. F. et al. Adjunctive statin therapy in patients with COVID-19: A systematic review analysis and meta-analysis of randomized controlled trials. Am. J. Med. https://doi.org/10.1016/j.amjmed.2024.06.002.) (2024).
Rong, Y. et al. Association of antecedent statin use on 30-day, 60-day and 90-day mortality among Mississippi Medicaid beneficiaries diagnosed with COVID-19. BMJ Open https://doi.org/10.1136/bmjopen-2023-076195 (2023).
Kuno, T., So, M., Iwagami, M., Takahashi, M. & Egorova, N. N. The association of statins use with survival of patients with COVID-19. J. Cardiol. 79, 494–500. https://doi.org/10.1016/j.jjcc.2021.12.012 (2022).
Wander, P. L. et al. Associations of statin use with 30-day adverse outcomes among 4 801 406 US Veterans with and without SARS-CoV-2: An observational cohort study. BMJ Open https://doi.org/10.1136/bmjopen-2021-058363 (2022).
Hunt, C. M. et al. Medications associated with lower mortality in a SARS-CoV-2 positive cohort of 26,508 veterans. J. Gen. Intern. Med. 37, 4144–4152. https://doi.org/10.1007/s11606-022-07701-3 (2022).
Rocheleau, G. L. Y. et al. Renin-angiotensin system pathway therapeutics associated with improved outcomes in males hospitalized with COVID-19. Crit. Care Med. 50, 1306–1317. https://doi.org/10.1097/CCM.0000000000005589 (2022).
Yin, J., Wang, C., Song, X., Li, X. & Miao, M. Effects of renin-angiotensin system inhibitors on mortality and disease severity of COVID-19 patients: A meta-analysis of randomized controlled Trials. Am. J. Hypertens. 35, 462–469. https://doi.org/10.1093/ajh/hpac001 (2022).
Jeffery MM, Oliveira J E Silva L, Bellolio F, Garovic VD, Dempsey TM, et al. Association of outpatient use of renin-angiotensin-aldosterone system blockers on outcomes of acute respiratory illness during the COVID-19 pandemic: a cohort study. BMJ Open, https://doi.org/10.1136/bmjopen-2021-060305. (2022).
Zhang, Z. et al. Long-term oral ACEI/ARB therapy is associated with disease severity in elderly COVID-19 omicron BA.2 patients with hypertension. BMC Infect. Dis. https://doi.org/10.1186/s12879-023-08913-6 (2023).
Little, S. J. Treatment of acute HIV infection and the potential role of acutely HIV-infected persons in cure studies. Top Antivir. Med. 23, 156–160 (2016).
Mulangu, S. et al. A randomized, controlled trial of ebola virus disease therapeutics. N. Engl. J. Med. https://doi.org/10.1056/NEJMoa1910993 (2019).
Bhavnani, D. et al. SARS-CoV-2 viral load is associated with risk of transmission to household and community contacts. BMC Infect Dis. 22, 672. https://doi.org/10.1186/s12879-022-07663-1 (2022).
Havlir, D. V. et al. HIV testing and treatment with the use of a community health approach in rural Africa. N. Engl. J. Med. 381, 219–229. https://doi.org/10.1056/NEJMoa1809866 (2019).
Zheng, S. et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ https://doi.org/10.1136/bmj.m1443 (2020).
Wang, G. et al. C-Reactive protein level may predict the risk of COVID-19 aggravation. Open Forum Infect Dis https://doi.org/10.1093/ofid/ofaa153 (2020).
Ghati, N. et al. Statin and aspirin as adjuvant therapy in hospitalised patients with SARS-CoV-2 infection: A randomised clinical trial (RESIST trial). BMC Infect Dis. 202, 606. https://doi.org/10.1186/s12879-022-07570-5 (2022).
Gaitan-Duarte, H. G. et al. Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with COVID-19: A pragmatic, open-label randomized trial. E Clin. Med. https://doi.org/10.1016/j.eclinm.2021.101242 (2022).
INSPIRATION-S Investigators. Atorvastatin versus placebo in patients with covid-19 in intensive care: randomized controlled trial. BMJ https://doi.org/10.1136/bmj-2021-068407 (2022).
The REMAP-CAP Investigators. Simvastatin in critically Ill patients with Covid-19. N. Engl. J. Med. 389(25), 2341–2354. https://doi.org/10.1056/NEJMoa2309995 (2023).
Makris, D. et al. Effect of pravastatin on the frequency of ventilator-associated pneumonia and on intensive care unit mortality: Open-label, randomized study. Crit. Care Med. 39, 2440–2446 (2011).
Papazian, L. et al. Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: A randomized clinical trial. JAMA https://doi.org/10.1001/jama.2013.280031 (2013).
Franco, R. et al. SARS-CoV-2 as a factor to disbalance the reninangiotensin system: a suspect in the case of exacerbated IL-6 production. J. Immunol. 205, 1198. https://doi.org/10.4049/jimmunol.2000642 (2020).
Duarte, M. et al. Telmisartan for treatment of Covid-19 patients: An open multicenter randomized clinical trial. E Clin. Med. https://doi.org/10.1016/j.eclinm.2021.100962 (2021).
Jardine, M. J. et al. Angiotensin receptor blockers for the treatment of covid-19: Pragmatic, adaptive, multicentre, phase 3, randomised controlled trial. BMJ https://doi.org/10.1136/bmj-2022-072175 (2022).
Anoh, E. A. et al. Subregional origins of emerging SARS-CoV-2 variants during the second pandemic wave in Côte d’Ivoire. Virus Genes 59, 370–376. https://doi.org/10.1007/s11262-023-01984-2 (2023).
Funding
ANRS/MIE, call Flash COVID pays du sud, 05th May 2020; Award number ECTZ 143960, The funder of the study (ANRS/MIE) played no role in the study design, data collection, data analysis, interpretation of outcomes, or report writing.
Author information
Authors and Affiliations
Contributions
FB was involved in the conception and study design, the data interpretation, and wrote the original draft of the manuscript. AD participated to the study design, data collection and data interpretation. VM was involved in the funding acquisition, the study design, the writing of the manuscript and managed the project. FE was involved in the literature search, funding acquisition, study design, data interpretation. PB analyzed and interpreted the virological data. CBA collected and analyzed the biological data. BTS collected and analyzed the biological data. LF supervised the project. AK implemented the software and managed the data. EA collected and interpreted the data. MD collected and interpreted the data. SN performed the data analysis and interpretation. VJ analyzed the data and participated to the first draft of the manuscript. SPE was involved in the conception and study design, the data interpretation. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
Fabrice Bonnet has received consulting honoraria, research grants, or both from Gilead Sciences, MSD, and ViiV Healthcare, outside the submitted work. Other authors have nothing to disclose. The other authors declare no competing interests.
Ethical approval
The trial was approved by the institutional national ethics committee (Comité National d’Ethique de la Vie et de la Santé (CNEVS), July 27, 2020) and was conducted in accordance with the 1964 Helsinki Declaration and the French and Ivorian regulations on clinical trials. We obtained written informed consent from all patients.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bonnet, F., Doumbia, A., Machault, V. et al. Atorvastatin and telmisartan do not reduce nasopharyngeal carriage of SARS-CoV-2 in mild or moderate COVID-19 in a phase IIb randomized controlled trial. Sci Rep 14, 25028 (2024). https://doi.org/10.1038/s41598-024-72449-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72449-1