Abstract
Background
We aimed to examine the efficacy of intravenous vitamin C (IV-VC) in the treatment of hospitalized patients with moderate or severe COVID-19.
Method
We conducted a single-center and retrospective study including patients with COVID-19 diagnosis who were hospitalized. Patients were categorized into three groups as those who received low-dose (LDVC group, 2 g/day, n = 183) or high-dose IV-VC (HDVC group, 25 g/day, n = 41) and who did not receive IV-VC (control group, n = 46).
Results
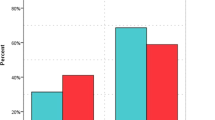
270 patients aged 19–97 years were enrolled. The median length of stay (LOS) was significantly high (9 days) in patients treated with high-dose VC when compared to patients treated with low-dose VC and control patients (6 vs 5 days, respectively). Need for intensive care unit (ICU) transfer was found to be significantly low in patients treated with low-dose VC (25.7%); contrarily, control patients had significantly higher rates of ICU transfer (67.4%), when compared to patients treated with high-dose VC (39%). Mortality of the LDVC group was significantly lower than that of the HDVC group (11.5 vs 29.3%). However, mortality rates were similar between the control and HDVC groups (21.7 vs 29.3%). According to the multivariate stepwise logistic regression mortality analysis, percent of change (∆%)-BUN was the most significant variable (p < 0.001), the second significant variable was ∆%-AST (p = 0.002), the third significant variable was respiratory distress (p = 0.002), and the fourth significant variable was the IV-VC groups (p = 0.017). The mortality risk of those in the LDVC group was 10.2 times low compared to the control group. Similarly, the risk of mortality in the HDVC group was 6.5 times lower than that of the control group.
Conclusion
Especially low and continious doses of IV-VC suggest fewer days of in-hospital LOS and survival benefit in hospitalized patients with moderate and severe COVID-19. Logistic regression analysis revealed that high-dose VC supplementation also had a mortality-reducing effect.
Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the confidentiality of health data with third parties, but are available from the corresponding author on reasonable request.
References
Ahnach M, Zbiri S, Nejjari S, Ousti F, Elkettani C (2020) C-reactive protein as an early predictor of COVID-19 severity. J Med Biochem 39(4):500–507. https://doi.org/10.5937/jomb0-27554
Arvinte C, Singh M, Marik PE (2020) Serum levels of vitamin C and vitamin D in a cohort of critically Ill COVID-19 patients of a north american community hospital intensive care unit in May 2020: a pilot study. Med Drug Discov 8:100064. https://doi.org/10.1016/j.medidd.2020.100064
Avila-Nava A, Pech-Aguilar AG, Lugo R, Medina-Vera I, Guevara-Cruz M, Gutiérrez-Solis AL (2022) Oxidative stress biomarkers and their association with mortality among patients infected with SARS-CoV-2 in mexico. Oxid Med Cell Longev 2022:1058813. https://doi.org/10.1155/2022/1058813
Belhadi, Chawki (2021) Acute Induced Scurvy: Implications for COVID-19 and the Cytokine Storm. Field Notes: A Journal of Collegiate Anthropology 12(4). Available at: https://dc.uwm.edu/fieldnotes/vol12/iss1/4
Boerenkamp LS, Gijsbers BLMG, Ververs EJ, Pijpers EMS, Spaetgens B, de Coninck A, Germeraad WTV, Wodzig WKWH, Wieten L, van Gorkom GNY, van Elssen CHMJ (2023) Low levels of serum and intracellular vitamin C in hospitalized COVID-19 patients. Nutrients 15(16):3653. https://doi.org/10.3390/nu15163653
Bogoch II, Watts A, Thomas-Bachli A, Huber C, Kraemer MUG, Khan K (2020) Potential for global spread of a novel coronavirus from China. J Travel Med. https://doi.org/10.1093/jtm/taaa011
Borrelli E, Roux-Lombard P, Grau GE, Girardin E, Ricou B, Dayer J, Suter PM (1996) Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med. https://doi.org/10.1097/00003246-199603000-00006
Cecchini R, Cecchini AL (2020) SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses 143:110102. https://doi.org/10.1016/j.mehy.2020.110102
Chiscano-Camón L, Ruiz-Rodriguez JC, Ruiz-Sanmartin A, Roca O, Ferrer R (2020) Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit Care 24(1):522. https://doi.org/10.1186/s13054-020-03249-y
De Grooth HJS, Spoelstra-de Man AME, Oudemans-van Straaten HM (2014) Early plasma vitamin C concentration, organ dysfunction and ICU mortality. intensive care medicine. Springer, New York
de Grooth HJ, Manubulu-Choo WP, Zandvliet AS, Spoelstra-de Man AME, Girbes AR, Swart EL, Oudemans-van Straaten HM (2018) vitamin C pharmacokinetics in critically Ill Patients: a randomized trial of four IV regimens. Chest 153(6):1368–1377. https://doi.org/10.1016/j.chest.2018.02.025
Domingo P, Mur I, Pomar V, Corominas H, Casademont J, de Benito N (2020) The four horsemen of a viral Apocalypse: The pathogenesis of SARS-CoV-2 infection (COVID-19). EBioMedicine 58:102887. https://doi.org/10.1016/j.ebiom.2020.102887
Doseděl M, Jirkovský E, Macáková K, Krčmová LK, Javorská L, Pourová J, Mercolini L, Remião F, Nováková L, Mladěnka P, Oemonom OBOT (2021) Vitamin C-sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients 13(2):615. https://doi.org/10.3390/nu13020615
Evans-Olders R, Eintracht S (2010) Hoffer LJ (2010) Metabolic origin of hypovitaminosis C in acutely hospitalized patients. Nutrition 26(11–12):1070–1074. https://doi.org/10.1016/j.nut.2009.08.015
Hemilä H, Chalker E (2023) Abrupt termination of vitamin C from ICU patients may increase mortality: secondary analysis of the LOVIT trial. Eur J Clin Nutr 77(4):490–494. https://doi.org/10.1038/s41430-022-01254-8
https://covid19.saglik.gov.tr/TR-66301/covid-19-rehberi.html. Accessed 03 June 2024
https://pubmed.ncbi.nlm.nih.gov/. Accessed 16 October 2024
https://www.worldometers.info/coronavirus/. Accessed 16 October 2024
Hudson EP, Collie JT, Fujii T, Luethi N, Udy AA, Doherty S, Eastwood G, Yanase F, Naorungroj T, Bitker L, Abdelhamid YA, Greaves RF, Deane AM, Bellomo R (2019) Pharmacokinetic data support 6-hourly dosing of intravenous vitamin C to critically ill patients with septic shock. Crit Care Resusc 21(4):236–242
JamaliMoghadamSiahkali S, Zarezade B, Koolaji S, SeyedAlinaghi S, Zendehdel A, Tabarestani M, Sekhavati Moghadam E, Abbasian L, Dehghan Manshadi SA, Salehi M, Hasannezhad M, Ghaderkhani S, Meidani M, Salahshour F, Jafari F, Manafi N, Ghiasvand F (2021) Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial. Eur J Med Res 26(1):20. https://doi.org/10.1186/s40001-021-00490-1
Karaaslan T, Karaaslan E (2022) Predictive value of systemic immune-inflammation index in determining mortality in COVID-19 patients. J Crit Care Med (Targu Mures) 8(3):156–164. https://doi.org/10.2478/jccm-2022-0013
Kow CS, Hasan SS, Ramachandram DS (2023) The effect of vitamin C on the risk of mortality in patients with COVID-19: a systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology 31(6):3357–3362. https://doi.org/10.1007/s10787-023-01200-5
Kumari P, Dembra S, Dembra P, Bhawna F, Gul A, Ali B, Sohail H, Kumar B, Memon MK, Rizwan A (2020) The role of vitamin C as adjuvant therapy in COVID-19. Cureus 12(11):e11779. https://doi.org/10.7759/cureus.11779
Long CL, Maull KI, Krishnan RS, Laws HL, Geiger JW, Borghesi L, Franks W, Lawson TC, Sauberlich HE (2003) Ascorbic acid dynamics in the seriously ill and injured. J Surg Res 109(2):144–148. https://doi.org/10.1016/s0022-4804(02)00083-5
Mahjoub L, Youssef R, Yaakoubi H, Salah HB, Jaballah R, Mejri M, Sekma A, Trabelsi I, Nouira S, Khrouf M, Soltane HB, Mezgar Z, Boukadida L, Zorgati A, Boukef R (2024) Melatonin, vitamins and minerals supplements for the treatment of Covid-19 and Covid-like illness: a prospective, randomized, double-blind multicenter study. Explore (NY) 20(1):95–100. https://doi.org/10.1016/j.explore.2023.06.009
Majidi N, Rabbani F, Gholami S, Gholamalizadeh M, BourBour F, Rastgoo S, Hajipour A, Shadnoosh M, Akbari ME, Bahar B, Ashoori N, Alizadeh A, Samipoor F, Moslem A, Doaei S, Suzuki K (2021) The effect of vitamin C on pathological parameters and survival duration of critically Ill coronavirus disease 2019 patients: a randomized clinical trial. Front Immunol 12:717816. https://doi.org/10.3389/fimmu.2021.717816
NIH COVID-19 Treatment Guideliness, https://www.covid19treatmentguidelines.nih.gov/. Accessed 03 June 2024
Olczak-Pruc M, Swieczkowski D, Ladny JR, Pruc M, Juarez-Vela R, Rafique Z, Peacock FW, Szarpak L (2022) Vitamin C supplementation for the treatment of COVID-19: a systematic review and meta-analysis. Nutrients. https://doi.org/10.3390/nu14194217
Pincemail J, Cavalier E, Charlier C, Cheramy-Bien JP, Brevers E, Courtois A, Fadeur M, Meziane S, Goff CL, Misset B, Albert A, Defraigne JO, Rousseau AF (2021) Oxidative stress status in COVID-19 patients hospitalized in intensive care unit for severe pneumonia. A Pilot Study Antioxidants (Basel) 10(2):257. https://doi.org/10.3390/antiox10020257
Ried K, BinJemain T, Sali A (2021) Therapies to prevent progression of COVID-19, including hydroxychloroquine, azithromycin, zinc, and vitamin D3 with or without intravenous vitamin C: an international, multicenter. Randomized Trial Cureus 13(11):e19902. https://doi.org/10.7759/cureus.19902
Sharma R, Patel A, Ojha T, Pablo LA, Vosoughi T, Ziegler C, Sivapragasam K, Pinto AD, Jenkins D, Hosseini B (2024) Role of antioxidant therapy in the treatment and prognosis of COVID-19: a systematic review and meta-analysis of randomized controlled trials. Curr Dev Nutr 8(5):102145. https://doi.org/10.1016/j.cdnut.2024.102145
Sinnberg T, Lichtensteiger C, Hill-Mündel K, Leischner C, Niessner H, Busch C, Renner O, Wyss N, Flatz L, Lauer UM, Hoelzle LE, Nohr D, Burkard M, Marongiu L, Venturelli S (2022) Vitamin C deficiency in blood samples of COVID-19 patients. Antioxidants (Basel) 11(8):1580. https://doi.org/10.3390/antiox11081580
Sinopoli A, Sciurti A, Isonne C, Santoro MM, Baccolini V (2024) The efficacy of multivitamin, vitamin A, vitamin B, vitamin C, and vitamin D supplements in the prevention and management of COVID-19 and long-COVID: an updated systematic review and meta-analysis of randomized clinical trials. Nutrients 16(9):1345. https://doi.org/10.3390/nu16091345
Smail SW, Babaei E, Amin K (2023) Hematological, inflammatory, coagulation, and oxidative/antioxidant biomarkers as predictors for severity and mortality in COVID-19: a prospective cohort-study. Int J Gen Med 16:565–580. https://doi.org/10.2147/IJGM.S402206
Sun L, Zhao JH, Fan WY, Feng B, Liu WW, Chen RQ, Ban C, Dang AG, Wang M, Luo KT, Zhou GY, Yu FF, Ba Y (2024) Therapeutic effects of high-dose vitamin C supplementation in patients with COVID-19: a meta-analysis. Nutr Rev 82(8):1056–1068. https://doi.org/10.1093/nutrit/nuad105
Uddin MS, Millat MS, Baral PK, Ferdous M, Uddin MG, Sarwar MS, Islam MS (2021) The protective role of vitamin C in the management of COVID-19: a review. J Egypt Public Health Assoc 96(1):33. https://doi.org/10.1186/s42506-021-00095-w
van Eijk LE, Tami A, Hillebrands JL, den Dunnen WFA, de Borst MH, van der Voort PHJ, Bulthuis MLC, Veloo ACM, Wold KI, Vincenti González MF, van der Gun BTF, van Goor H, Bourgonje AR (2021) Mild coronavirus disease 2019 (COVID-19) is marked by systemic oxidative stress: a pilot study. Antioxidants (Basel) 10(12):2022. https://doi.org/10.3390/antiox10122022
Victor VM, Guayerbas N, Puerto M, De la Fuente M (2001) Changes in the ascorbic acid levels of peritoneal lymphocytes and macrophages of mice with endotoxin-induced oxidative stress. Free Radic Res 35(6):907–916. https://doi.org/10.1080/10715760100301401
Yang AP, Liu JP, Tao WQ, Li HM (2020) The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol 84:106504. https://doi.org/10.1016/j.intimp.2020.106504
Zhang J, Rao X, Li Y, Zhu Y, Liu F, Guo G, Luo G, Meng Z, De Backer D, Xiang H, Peng Z (2021) Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care 11(1):5. https://doi.org/10.1186/s13613-020-00792-3
Acknowledgements
The authors acknowledge the participating institutions and investigators.
Funding
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The first draft of the manuscript was written by Burak Uz and all authors commented on previous versions of the manuscript. Data collection and literature search were performed by Burak Uz, Özgür İnce and Can Gümüş; the tables were designed by Burak Uz; statistical analysis of the manuscript were performed by Emrah Gökay Özgür and Gülnaz Nural Bekiroğlu. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Ondokuz Mayıs University (13.04.2022/OMÜ KAEK 2022-167). In addition, this trial was approved by the Academic and Ethichs Committee of Medicana International Samsun Hospital (03.03.2022/2) and the General Directorate of Health Services at the Republic of Turkey Ministry of Health (16.03.2022).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Our manuscript does not contain any individual person’s data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uz, B., İnce, Ö., Gümüş, C. et al. Role of intravenous vitamin C on outcomes in hospitalized patients with moderate or severe COVID-19: a real life data of Turkish patients. Inflammopharmacol 33, 833–843 (2025). https://doi.org/10.1007/s10787-024-01597-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-024-01597-7