Abstract
Background
Aesthetic injections have become increasingly popular for maintaining a youthful appearance. However, with the rise of SARS-CoV-2, there have been concerns about potential complications. This study aims to summarize and understand the complications that occur in individuals who have received cosmetic injections after SARS-CoV-2 infection or vaccination. By doing so, we hope to provide recommendations to minimize these complications and ensure the safety of aesthetic treatments in the current COVID-19 era.
Methods
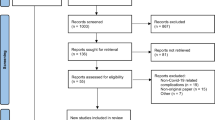
The PRISMA guidelines, the Preferred Reporting Program for Systematic Reviews and Meta-Analyses, were used for this review. Databases including PubMed, EMBASE, Medline, Web of Science and ScienceDirect were searched. The last search time of each database was May 10, 2023. In addition, relevant references were manually searched.
Results
A total of 26 studies containing 139 patients were searched. The complication with the highest percentage of reported patients was delayed inflammatory response (DIR) (n = 68; 48.92%), followed by diminished efficacy (n = 45; 32.37%) and filler reaction (n = 12; 8.63%). The remaining complications include hypersensitivity reactions, symptomatic hypercalcemia, sub-acute hypersensitive reactions, hyperalgesia, infection, fat necrosis and granulomatous reaction.
Conclusions
Cosmetic injectable procedures are generally safe but may have adverse effects, particularly during the pandemic. It is important for individuals to fully understand these risks beforehand. Clinicians should be knowledgeable about adverse event mechanisms and management to prevent issues. Industry leaders should strengthen risk management efforts to ensure safe and steady development of cosmetic injections. Overall, a comprehensive understanding, effective communication and risk management are crucial for the safe use of cosmetic injectable procedures.
Level of Evidence III
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors at www.springer.com/00266.


Similar content being viewed by others
Availability of Data and Material
All data analyzed during this study are included in this manuscript. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- COVID-19:
-
Coronavirus disease 19
- ACE2:
-
Angiotensin-converting enzyme 2
- BTA:
-
Botulinum toxin A
- HA:
-
Hyaluronic acid
- DIR:
-
Delayed inflammatory response
- SES:
-
Silicone embolism syndrome
References
Hu B, Guo H, Zhou P, Shi ZL (2021) Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19(3):141–154
World Health Organization (2023) Covid-19 weekly epidemiological update, 124 edn
Kevadiya BD, Machhi J, Herskovitz J et al (2021) Diagnostics for sars-cov-2 infections. Nat Mater 20(5):593–605
Guan WJ, Ni ZY, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720
Zhao W, Li H, Li J, Xu B, Xu J (2022) The mechanism of multiple organ dysfunction syndrome in patients with covid-19. J Med Virol 94(5):1886–1892
Jackson CB, Farzan M, Chen B, Choe H (2022) Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 23(1):3–20
Chen M, Shen W, Rowan NR et al (2020) Elevated ace-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Respir J 56(3):2001948
Merad M, Blish CA, Sallusto F, Iwasaki A (2022) The immunology and immunopathology of COVID-19. Science 375(6585):1122–1127
Paprottka FJ, Rolfes SB, Richter DF, Kaye KO (2021) Covid-19 pandemic: evaluation of socio-economic impact on aesthetic plastic surgery providers. Aesthet Plast Surg 45(4):1877–1887
Chen Y, Zhou S (2023) Impacts of video communication on psychological well-being and cosmetic surgery acceptance. Comput Human Behav 141:107625
Pang R, Wei Z, Liu W et al (2020) Influence of the pandemic dissemination of COVID-19 on facial rejuvenation: a survey of Twitter. J Cosmet Dermatol 19(11):2778–2784
Abdolalizadeh P, Kashkouli MB, Jafarpour S, Rezaei S, Ghanbari S, Akbarian S (2023) Impact of COVID-19 on the patient referral pattern and conversion rate in the university versus private facial plastic surgery centers. Int Ophthalmol 43(3):707–715
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Akdogan N (2021) Severe hyperalgesia and pain during botulinum toxin injection avoiding application in a patient 1 week after COVID-19 infection. J Cosmet Dermatol 20(3):755–756
Alijotas-Reig J, Garcia-Glmenez V, Velthuis PJ, Niessen FB, Decates TS (2022) Inflammatory immune-mediated adverse reactions induced by COVID-19 vaccines in previously injected patients with soft tissue fillers: a case series of 20 patients. J Cosmet Dermatol 21(8):3181–3187
Azzouz S, Lanoue D, Champagne K, Genest G (2023) Delayed hypersensitivity reaction to cosmetic filler following two COVID-19 vaccinations and infection. Allergy Asthma Clin Immunol 19(1):31
Beamish IV, Bogoch II, Carr D (2022) Delayed inflammatory reaction to dermal fillers after COVID-19 vaccination: a case report. CJEM 24(4):444–446
Belluco PES, Lima CRF, Belluco RZF et al (2023) Delayed hypersensitivity reaction to hyaluronic acid dermal filler following ChAdOx1-S recombinant vaccine against coronavirus: case report. Allergy Eur J Allergy Clin Immunol 78:335–336
Brau-Javier CNN, Caro-Muniz AP, Canizares O (2023) Facial fat necrosis after autologous fat transfer possibly associated with SARS-CoV-2 vaccine. J Cosmet Dermatol 22(5):1477–1480
Bray A, Reyes JVM, Tarlin N, Stern A (2021) Case series: hypercalcemia from granulomatous silicosis developing after covid-19 infection. J Investig Med High Impact Case Rep 9:23247096211051210
Calvisi L (2022) Hyaluronic acid delayed inflammatory reaction after third dose of SARS-CoV-2 vaccine. J Cosmet Dermatol 21(6):2315–2317
Guo X, Li T, Wang Y, Jin X (2021) Sub-acute hypersensitive reaction to botulinum toxin type a following covid-19 vaccination: case report and literature review. Medicine (United States) 100(49):e27787
Hamed Azzam S, Mukari A, Hamed M, Kridin K (2022) Influence of covid-19 mRNA vaccination on the efficacy and safety of botulinum toxin type a injections. J Cosmet Dermatol 21(9):3663–3666
Incel Uysal P, Gunhan O (2022) Granulomatous reaction at PRP/fat injection sites after recovering from SARS-Co-V2: a case report. J Cosmet Dermatol 21(2):426–428
Jeon HB (2022) Midface infection after COVID-19 vaccination in a patient with calcium hydroxylapatite dermal filler: a case report and literature review. Arch Plast Surg 49(3):310–314
Li Z, Zhao P, Xu Q, Bi J, Huo R (2023) Delayed inflammatory reactions after hyaluronic acid filling of neck lines: a case report. Clin Cosmet Investig Dermatol 16:99–102
Lopez PV, Garcia PT, Lopez-Pitalua JA, Pinto H (2023) Side effects after hyaluronic acid facial injection in adults during COVID-19 pandemic. J Cosmet Dermatol 22(6):1714–1719
McMahon DE, Amerson E, Rosenbach M et al (2021) Cutaneous reactions reported after Moderna and Pfizer covid-19 vaccination: a registry-based study of 414 cases. J Am Acad Dermatol 85(1):46–55
Michon A (2021) Hyaluronic acid soft tissue filler delayed inflammatory reaction following covid-19 vaccination—a case report. J Cosmet Dermatol 20(9):2684–2690
Michon A (2022) Ace inhibitors—an effective treatment for hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID-19 vaccination. J Cosmet Dermatol 21(4):1369–1370
Munavalli GG, Guthridge R, Knutsen-Larson S, Brodsky A, Matthew E, Landau M (2022) COVID-19/SARS-CoV-2 virus spike protein-related delayed inflammatory reaction to hyaluronic acid dermal fillers: a challenging clinical conundrum in diagnosis and treatment. Arch Dermatol Res 314(1):1–15
Munavalli GG, Knutsen-Larson S, Lupo MP, Geronemus RG (2021) Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination-a model for inhibition of angiotensin II–induced cutaneous inflammation. JAAD Case Rep 10:63–68
Naouri M, Dahan S, Prost ALP et al (2023) Good tolerance of hyaluronic acid filler injections during the COVID-19 pandemic. J Cosmet Dermatol 22(2):342–346
Oliver SE, Gargano JW, Marin M et al (2021) The advisory committee on immunization practices’ interim recommendation for use of moderna COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep 69(5152):1653–1656
Rowland-Warmann MJ (2021) Hypersensitivity reaction to hyaluronic acid dermal filler following novel coronavirus infection—a case report. J Cosmet Dermatol 20(5):1557–1562
Safir A, Samuelov L, Sprecher E, Daniely D, Artzi O (2022) Association between BNT162b2 vaccination and the development of delayed inflammatory reactions to hyaluronic acid-based dermal fillers-a nationwide survey. J Cosmet Dermatol 21(10):4107–4113
Savva D, Battineni G, Amenta F, Nittari G (2021) Hypersensitivity reaction to hyaluronic acid dermal filler after the Pfizer vaccination against SARS-CoV-2. Int J Infect Dis 113:233–235
Shome D, Doshi K, Vadera S, Kapoor R (2021) Delayed hypersensitivity reaction to hyaluronic acid dermal filler post-COVID-19 viral infection. J Cosmet Dermatol 20(5):1549–1550
Virdi G (2022) Dermal fillers and COVID-19: angioedema with urticaria in a patient post COVID-19 infection. Cureus 14(4):e24461
Rauso R, Lo Giudice G, Zerbinati N, Nicoletti GF, Fragola R, Tartaro G (2021) Adverse events following COVID-19 vaccine in patients previously injected with facial filler: scoping review and case report. Appl Sci 11(22):10888
Ng CL, Tay EY, D'Souza AR (2023) Localised swelling at sites of dermal filler injections following administration of Covid-19 vaccines: a systematic review. Singapore Med J SMJ-2021-157
Kalantari Y, Aryanian Z, Mirahmadi SMS, Alilou S, Hatami P, Goodarzi A (2022) A systematic review on COVID-19 vaccination and cosmetic filler reactions: a focus on case studies and original articles. J Cosmet Dermatol 21(7):2713–2724
Bachour Y, Bekkenk MW, Rustemeyer T, Kadouch JA (2022) Late inflammatory reactions in patients with soft tissue fillers after SARS-CoV-2 infection and vaccination: a systematic review of the literature. J Cosmet Dermatol 21(4):1361–1368
Guo X, Li T, Wang Y, Jin X (2021) Sub-acute hypersensitive reaction to botulinum toxin type a following covid-19 vaccination: Case report and literature review. Medicine (Baltimore) 100(49):e27787
Kato K, Inoue E, Tanaka S, Kawamoto H (2022) Increase in the incidence of acute inflammatory reactions to injectable fillers during COVID-19 era. J Cosmet Dermatol 21(5):1816–1821
Kalantari Y, Sadeghzadeh-Bazargan A, Aryanian Z, Hatami P, Goodarzi A (2022) First reported case of delayed-type hypersensitivity reaction to non-hyaluronic acid polycaprolactone dermal filler following COVID-19 vaccination: a case report and a review of the literature. Clin Case Rep 10(2):e05343
Jeon HB, Yoon JH, Lim NK (2022) Midface infection after COVID-19 vaccination in a patient with calcium hydroxylapatite dermal filler: a case report and literature review. Arch Plast Surg 49(3):310–314
(2022) Aesthetic plastic surgery national databank statistics 2020-2021. Aesthet Surg J 42(Suppl 1):1–18
Liu W, Wei Z, Cheng X, Pang R, Zhang H, Li G (2021) Public interest in cosmetic surgical and minimally invasive plastic procedures during the covid-19 pandemic: infodemiology study of twitter data. J Med Internet Res 23(3):e23970
Cristel RT, Gandhi ND, Issa TZ, Kola E, Demesh D, Dayan SH (2021) A randomized, single-blind, crossover study evaluating the impact of onabotulinumtoxina treatment on mood and appearance during the covid-19 pandemic. Aesthet Surg J 41(9):1199–1205
Guarino E (2023) Botulinum toxin in the oily skin: advantage of a multi-needle device for a controlled release. J Drugs Dermatol 22(1):41–44
Goodman GJ, Liew S, Callan P et al (2020) Re facial aesthetic injections in clinical practice: pretreatment and post-treatment consensus recommendations to minimise adverse outcome region-specific changes in line with the COVID-19 pandemic. Australas J Dermatol 61(4):362–366
Soares FHC, Kubota GT, Fernandes AM et al (2021) Prevalence and characteristics of new-onset pain in COVID-19 survivours, a controlled study. Eur J Pain 25(6):1342–1354
Zhou YQ, Liu Z, Liu ZH et al (2016) Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation 13(1):141
Fernández-de-Las-Peñas C, Navarro-Santana M, Plaza-Manzano G, Palacios-Ceña D, Arendt-Nielsen L (2022) Time course prevalence of post-COVID pain symptoms of musculoskeletal origin in patients who had survived severe acute respiratory syndrome coronavirus 2 infection: a systematic review and meta-analysis. Pain 163(7):1220–1231
Lacroix-Desmazes S, Mouly S, Popoff M-R, Colosimo C (2017) Systematic analysis of botulinum neurotoxin type a immunogenicity in clinical studies. Basal Ganglia 9:12–17
Witmanowski H, Błochowiak K (2020) The whole truth about botulinum toxin—a review. Postepy Dermatol Alergol 37(6):853–861
Bellows S, Jankovic J (2019) Immunogenicity associated with botulinum toxin treatment. Toxins (Basel) 11(9):491
Yeh YT, Peng JH, Peng HP (2018) Literature review of the adverse events associated with botulinum toxin injection for the masseter muscle hypertrophy. J Cosmet Dermatol 17(5):675–687
Baizabal-Carvallo JF, Jankovic J, Feld J (2013) Flu-like symptoms and associated immunological response following therapy with botulinum toxins. Neurotox Res 24(2):298–306
George EB, Cotton AC, Shneyder N, Jinnah HA (2018) A strategy for managing flu-like symptoms after botulinum toxin injections. J Neurol 265(8):1932–1933
DeVictor S, Ong AA, Sherris DA (2021) Complications secondary to nonsurgical rhinoplasty: A systematic review and meta-analysis. Otolaryngol Head Neck Surg 165(5):611–616
Grabska-Zielińska S, Sionkowska A (2021) How to improve physico-chemical properties of silk fibroin materials for biomedical applications?-Blending and cross-linking of silk fibroin-a review. Materials (Basel) 14(6):1510
Artzi O, Cohen JL, Dover JS et al (2020) Delayed inflammatory reactions to hyaluronic acid fillers: a literature review and proposed treatment algorithm. Clin Cosmet Investig Dermatol 13:371–378
Marusza W, Olszanski R, Sierdzinski J et al (2019) Treatment of late bacterial infections resulting from soft-tissue filler injections. Infect Drug Resist 12:469–480
Alli N, Murdoch M, Meer S (2022) Delayed adverse reaction to a natural dermal filler mimicking salivary gland neoplasia. Bull Natl Res Cent 46(1):97
Theoharides TC (2020) The impact of psychological stress on mast cells. Ann Allergy Asthma Immunol 125(4):388–392
Finlay BB, Amato KR, Azad M et al (2021) The hygiene hypothesis, the COVID pandemic, and consequences for the human microbiome. Proc Natl Acad Sci USA 118(6):e2010217118
Anthony Cleland D, Tsai CHH, Vo J, Moretta D (2022) COVID-19 infection or buttock injections? The dangers of aesthetics and socializing during a pandemic. J Crit Care Med (Targu Mures) 8(1):49–54
Madan N, Khan U, Martins A et al (2022) Recurrent silicone embolism syndrome requiring va ecmo. Respir Med Case Rep 36:101576
Munavalli GG, Knutsen-Larson S, Lupo MP, Geronemus RG (2021) Oral angiotensin-converting enzyme inhibitors for treatment of delayed inflammatory reaction to dermal hyaluronic acid fillers following COVID-19 vaccination-a model for inhibition of angiotensin II-induced cutaneous inflammation. JAAD Case Rep 10:63–68
Turkmani MG, De Boulle K, Philipp-Dormston WG (2019) Delayed hypersensitivity reaction to hyaluronic acid dermal filler following influenza-like illness. Clin Cosmet Investig Dermatol 12:277–283
Gotkin RH, Gout U, Sattler S et al (2021) Global recommendations on covid-19 vaccines and soft tissue filler reactions: a survey-based investigation in cooperation with the international society for dermatologic and aesthetic surgery (ISDS). J Drugs Dermatol 20(4):374–378
El-Boghdadly K, Cook TM, Goodacre T et al (2021) SARS-CoV-2 infection, COVID-19 and timing of elective surgery: a multidisciplinary consensus statement on behalf of the association of anaesthetists, the Centre for Peri-operative Care, the Federation of Surgical Specialty Associations, the Royal College of Anaesthetists and the Royal College of Surgeons of England. Anaesthesia 76(7):940–946
Brusini R, Iehl J, Clerc E, Gallet M, Bourdon F, Faivre J (2022) Comparative preclinical study of lidocaine and mepivacaine in resilient hyaluronic acid fillers. Pharmaceutics 14(8):1553
Calomeni M, Bravo BSF, Schelke LW et al (2023) Precision of soft-tissue filler injections: an ultrasound-based verification study. Aesthet Surg J 43(3):353–361
Alam M, Kakar R, Dover JS et al (2021) Rates of vascular occlusion associated with using needles vs cannulas for filler injection. JAMA Dermatol 157(2):174–180
Oshima M, Deitiker PR, Jankovic J, Duane DD, Aoki KR, Atassi MZ (2011) Human t-cell responses to botulinum neurotoxin. Responses in vitro of lymphocytes from patients with cervical dystonia and/or other movement disorders treated with BoNT/A or BoNT/B. J Neuroimmunol 240–241:121–128
Field M, Splevins A, Picaut P et al (2018) Abobotulinumtoxina (dysport(®)), onabotulinumtoxina (botox(®)), and incobotulinumtoxina (xeomin(®)) neurotoxin content and potential implications for duration of response in patients. Toxins (Basel) 10(12):535
Alijotas-Reig J, Fernández-Figueras MT, Puig L (2013) Inflammatory, immune-mediated adverse reactions related to soft tissue dermal fillers. Semin Arthr Rheum 43(2):241–258
Funding
This study was funded by Cultivate Innovation Fund of the Sixth Medical Center of PLA General Hospital (CXPY202123).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict interests.
Ethics Approval and Consent to Participate
All analyses were based on previously published studies; hence, no ethical approval and patient consent were required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1. Search strategy
Appendix 1. Search strategy
The combined text and medical subject heading (MeSH) terms used were: “covid” and “injection.”
PubMed
The database was searched on May 10, 2023, n = 166.
Search Strategy:
(covid OR SARS-CoV-2 OR coronavirus) AND (injection OR inject) AND (aesthetic OR cosmetic OR plastic OR filler OR toxin OR fat)
Web of Science
The database was searched on May 10, 2023, n = 193.
Search Strategy:
-
1.
TOPIC: (“covid” OR “SARS-CoV-2” OR “coronavirus”)
-
2.
TOPIC: (“injection” OR “inject”)
-
3.
TOPIC: (“aesthetic” OR “cosmetic” OR “plastic” OR “filler” OR “toxin” OR “fat”)
-
4.
4 #1 AND #2 AND #3
EMBASE
The database was searched on May 10, 2023, n = 191.
Search Strategy:
-
1.
covid (396323)
-
2.
SARS-CoV-2 (145783)
-
3.
coronavirus (375737)
-
4.
injection (820884)
-
5.
inject (13722)
-
6.
aesthetic (65983)
-
7.
cosmetic (96735)
-
8.
plastic (372147)
-
9.
filler (15896)
-
10.
toxin (218648)
-
11.
fat (504646)
-
12.
#1 OR #2 OR #3 (434457)
-
13.
#4 OR #5 (827512)
-
14.
#6 OR #7 OR #8 OR #9 OR #10 OR #11 (1193041)
-
15.
#12 AND #13 AND #14 (191)
Ovid MEDLINE
The database was searched on May 10, 2023, n = 84.
Search Strategy:
-
1.
covid (23463)
-
2.
SARS-CoV-2 (27210)
-
3.
coronavirus (18826)
-
4.
injection (10026)
-
5.
injection (9862)
-
6.
aesthetic (6547)
-
7.
cosmetic (6979)
-
8.
plastic (10129)
-
9.
filler (4879)
-
10.
toxin (11040)
-
11.
fat (11799)
-
12.
“covid” OR “SARS-CoV-2” OR “coronavirus”
-
13.
“injection” OR “inject”
-
14.
“aesthetic” OR “cosmetic” OR “plastic” OR “filler” OR “toxin” OR “fat”
-
15.
12 and 13 and 14
ScienceDirect
The database was searched on May 10, 2023, n = 166.
Search Strategy:
Title, abstract, keywords: ((“covid” OR “SARS-CoV-2” OR “coronavirus”) and (“injection” OR “inject”) and (“aesthetic” OR “cosmetic” OR “plastic” OR “filler” OR “toxin” OR “fat”))
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, Q., Zhang, P., Zhou, G. et al. Impact of SARS-CoV-2 Vaccination or Infection on the Safety and Efficacy of Aesthetic Injections: A Systematic Review. Aesth Plast Surg (2024). https://doi.org/10.1007/s00266-023-03769-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00266-023-03769-2