Abstract
The coronavirus-2 has led to a global pandemic of COVID-19 with an outbreak of severe acute respiratory syndrome leading to worldwide quarantine measures and a rise in death rates. The objective of this study is to propose a green, sensitive, and selective densitometric method to simultaneously quantify remdesivir (REM) in the presence of the co-administered drug linezolid (LNZ) and rivaroxaban (RIV) in spiked human plasma. TLC silica gel aluminum plates 60 F254 were used as the stationary phase, and the mobile phase was composed of dichloromethane (DCM): acetone (8.5:1.5, v/v) with densitometric detection at 254 nm. Well-resolved peaks have been observed with retardation factors (Rf) of 0.23, 0.53, and 0.72 for REM, LNZ, and RIV, respectively. A validation study was conducted according to ICH Q2 (R1) Guidelines. The method was rectilinear over the concentration ranges of 0.2–5.5 μg/band, 0.2–4.5 μg/band and 0.1–3.0 μg/band for REM, LNZ and RIV, respectively. The sensitivities of REM, LIN, and RIV were outstanding, with quantitation limits of 128.8, 50.5, and 55.8 ng/band, respectively. The approach has shown outstanding recoveries ranging from 98.3 to 101.2% when applied to pharmaceutical formulations and spiked human plasma. The method’s greenness was assessed using Analytical Eco-scale, GAPI, and AGREE metrics.
Similar content being viewed by others
Introduction
By the end of 2019, abnormal pneumonia outbreak associated with a new coronavirus had begun in Wuhan City, China. Afterwards it was termed Covid-19 by The World Health Organization (WHO). The disease spread so rapidly that the WHO declared it a pandemic in March 20201. The main feature of SARS-CoV-2 is its nonspecific symptoms, which are frequently misdiagnosed with flu and common cold2.
The other troubling aspect of this condition is its unknown prognosis, which can result in deadly outcomes like pneumonia and acute respiratory syndrome, or produce none to mild respiratory tract complaints which will subside just with supportive care. Patients with diabetes, chronic respiratory and cardiovascular disorders, cancer, and immune system deficiency may encounter serious pathological complications that result in fatality3.
In the absence of a specific antiviral medication against SARS-CoV-2, along with the failure of mass vaccination to halt the pandemic4, studies were carried out to evaluate the efficiency of existing antiviral agents targeting SARS-Cov2 such as remdesivir5.
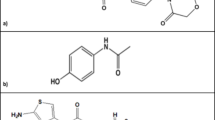
Remdesivir (REM) is 2-ethylbutyl (2S)-2-[[[(2R,3S,4R,5R)-5-(4-amino pyrrolo[2,1-f][1,2,4]triazin-7-yl)-5cyano3,4dihydroxyoxolanyl]methoxyphenoxy phosphoryl] amino] propanoate, (Fig. 1a). It was originally developed as an Ebola virus treatment by Gilead Sciences6.
Remdesivir is a nucleotide analog prodrug, that disrupts the viral RNA synthesis by being bioactivated into GS-441524 and phosphorylated into an active nucleoside triphosphate metabolite7.
Studies explored REM’s effects on SARS-CoV-2 as a potential COVID-19 treatment. The FDA approved REM as the sole antiviral treatment for COVID-19 due to its ability to block the virus’s replication8.
The FDA has accorded REM an “emergency use authorization” for severe COVID-19 since May 1, 2020, though few analytical methods has been reported for the estimation of remdesivir including, liquid chromatographic techniques9,10,11,12,13,14,15,16,17, electrochemical approaches18, spectrophotometric17,19 and spectrofluorimetric methods20,21,22. Most already elaborated methods are time-consuming, require expensive analytical tools, hazardous chemicals, and skilled personnel. The present work aimed to determine REM using a simple analytical procedure.
In order to limit the viral replication and improve the patients’ health, COVID-19 treatment involved the administration of many therapeutics from various categories, trying many preexisting medicines23. Therefore, a quick, valid, and reasonably priced bioanalytical approach for REM measurement in the human plasma matrix is urgently required for use in further clinical investigation and therapeutic drug monitoring.
The treatment guidance includes the use of broad-spectrum antibiotics for the treatment of secondary bacterial infection and acquired pneumonia24. Linezolid (LNZ) is (S)-N-[[3-[3-Fluoro-4-(4-morpholinyl)phenyl]-2-oxo-5-oxazolidinyl] methyl] acetamide (Fig. 1b), an antibiotic, was effective in treating COVID-19 patients with bacterial pneumonia, linezolid’s superiority is a result of its better penetration into the respiratory secretion compared to other broad-spectrum antibiotics, particularly vancomycin25. In addition, molecular docking studies have demonstrated the interaction between linezolid and the SARS-CoV-2 spike protein, indicating the stability and potential activity of linezolid on the SARS-CoV-2 protein26.
Anticoagulants are also included in the treatment protocol to reduce the risk of thrombosis, which is observed in nearly all COVID-19 patients27. Rivaroxaban (RIV) is (S)-5-chloro-N-{[2-oxo-3-[4-(3-oxomorpholin-4-yl) phenyl]oxazolidin-5-yl]methyl}vthiophene-2-carboxamide (Fig. 1c), which reduces the risk of venous thromboembolism without significantly raising the risk of severe bleeding, resulting in a net therapeutic benefit. This benefit seems to be more noticeable in patients whose initial hospitalization was due to an infectious condition, particularly pneumonia28.
The US Food and Drug Administration (FDA) granted authorization to remdesivir, as the initial antiviral medication approved for treating COVID-19. Antibiotics, antipyretics, corticosteroids, and anticoagulants are also included in the treatment protocol, which primarily relies on remdesivir. There is currently no reported method for simultaneously analyzing REM, LNZ, and RIV in biological fluids as co-administered medications. Thus, the current study aims at developing a simple, cost-effective, and eco-friendly analytical technique for estimating the licensed COVID-19 antiviral drug REM in plasma in the presence of routinely used drugs in corona virus treatment: antibiotics such as LNZ and anticoagulants such as RIV.
Experimental
Apparatus
-
CAMAG® TLC scanner 3 (CAMAG, Muttenz, Switzerland) and winCATS® software. The scanner uses a deuterium lamp as the radiation source with scanning speed of 20 mm/s. Absorbance mode was utilized as the scan mode and the output is a chromatogram and an integrated peak area.
-
Linomat 5 autosampler with 100 µL microsyringe (CAMAG, Muttenz, Switzerland)
-
TLC Silica gel 60 F254 (Aluminum sheets 20 × 20 cm, 0.1 mm thickness, Merck, Darmstadt, Germany).
-
Vortex mixer, ZX3 (Velp. Scientifca, Usmate, Italy).
-
Centrifuge, model 2-16P (Sigma, Osterode am Harz, Germany).
Materials and reagents
-
Remdesivir reference substance (Purity 99.8% as certified) was generously gifted by EIPICo. 10th of Ramadan City, Egypt.
-
Linezolid reference substance (Purity 99.8% as certified) was kindly provided by Averroes Pharma, 6th Industrial Zone Sadat City, Egypt.
-
Rivaroxaban reference substance (Purity 99.6% as certified) was kindly supplied as a gift sample from AChemBlock, Burlingame, USA.
-
HPLC grade methanol, ethanol and ethyl acetate were purchased from Fisher Scientifics, Belgium.
-
Dichloromethane of analytical grade was obtained from Pioneers for chemicals (Piochem, 6th October City, Egypt).
-
Human plasma samples were supplied by Mansoura University Hospital, Mansoura, Egypt, and kept in the freezer at − 20 °C until usage.
Pharmaceutical dosage forms
Remdesivir-Rameda® concentrate for solution for I.V infusion, batch number 214188 (Rameda Pharmaceuticals, 6th October City, Giza, Egypt) stated to contain 100.0 mg REM/vial, was obtained from the Egyptian market.
Linezolid® solution for IV infusion, batch number 21080023 (Global NAPI Pharmaceuticals, 6th October City, Giza, Egypt), was obtained from the Egyptian market and was noted to contain 200.0 mg/100 mL-vial.
Xarelto® film coated tablets (Bayer Schering Pharma, batch no. 04008500074763) were bought as well from the Egyptian market and stated to contain 10.0 mg/tablet of rivaroxaban.
Standard solutions
Accurately weighed amounts equivalent to 10.0 mg of REM, LNZ and RIV were put into three separate 10 mL volumetric flask then the volumes were brought up to the required volume by methanol for REM and LNZ and acetonitrile for RIV to produce 1.0 mg/mL standard solutions. The produced stock solutions remained stable for 14 days in the refrigerator without changing.
Procedures
Chromatographic conditions
Following the saturation of the chromatographic jar for 30 min by the eluent system; dichloromethane–acetone (8.5:1.5, v/v). At 1.0 cm from the bottom edge, the sample bands were applied. Subsequently, chromatographic development and air drying was performed. This was followed by scanning the plates at 254 nm.
Calibration graphs construction
In methanol, REM, LNZ, and RIV concentration sets were prepared, with concentrations ranging from 20 to 550, 20 to 450, and 10 to 300 µg/mL, respectively. In triplicates, 10 µL aliquots of each flask were spotted on TLC plates to obtain concentration ranges of 0.2–5.5, 0.2–4.5, and 0.1–3.0 µg/band of REM, LNZ, and RIV, respectively. By graphing concentrations (µg/band) versus the relevant areas under the peaks, calibration plots were constructed.
Pharmaceutical dosage forms analysis
An accurately measured volume of the Remdesivir-Rameda® concentrate for solution for I.V infusion equivalent to 10.0 mg was transferred into a 10-mL volumetric flask, diluted with 5 mL methanol, shaken thoroughly, and then completed to the mark using the same solvent.
An accurately measured volume of Linezolid I.V solution equivalent to 10.0 mg was transferred into a 10 mL volumetric flask, 5 mL methanol was added, shaken well, and diluted to the required volume using the same solvent.
Accurately weighed ten Xarelto® film-coated tablets were pulverized into fine powder then a weighed quantity equivalent to 10.0 mg of rivaroxaban was transferred into a 10 mL volumetric flask. 5 mL of acetonitrile was added, and the solution was sonicated for 10 min. Using the same solvent, the volume was completed to 10 mL, then the solution was filtered using a 0.45 µm membrane filter.
To prepare working solutions, serial dilutions of each stock solution (1.0 mg/mL) were diluted with methanol. The corresponding regression equations for each drug were used to estimate the % recoveries of REM, LNZ, and RIV.
Spiked human plasma
Human plasma samples of 1.0 mL were transferred to 15 mL centrifuge falcon tubes, spiked with various concentrations of the studied drugs. After completing the volume to 5 mL with acetonitrile as a protein precipitating agent, the solutions were vortexed for 1 min before centrifuging at 3600 rpm for 30 min. Concentration ranges of 20–550, 20–450, and 10–300 µg/mL for REM, LNZ, and RIV, respectively, was obtained by transferring 1.0 mL of clear supernatants into 10 mL volumetric flasks and making up to the mark with methanol. REM, LNZ, and RIV concentration ranges of 0.20–5.50, 0.20–4.50, and 0.1–3.00 µg/band, respectively, were obtained by applying 10 µL aliquots of each concentration flask onto the TLC plates in triplicate. A blank experiment was run, and calibration graphs were generated concurrently.
Ethics approval
There are no human subjects in this research and informed consent is not applicable. The used plasma is pooled plasma from the blood bank of Mansoura university hospital.
Results and discussion
Rapid and economic TLC- densitometric technique was optimized to develop a validated, sensitive, and highly selective method for the estimation of REM, LNZ and RIV with minimal environmental damage. The proposed TLC approach has the plus of simultaneously determining multi analytes with a very straightforward sample preparation process and low solvent consumption. Notably the proposed method offers several advantages over previously reported traditional HPLC techniques17,29,30,31,32,33, regarding its simplicity, cost-effectiveness, and rapid analysis time. Providing a promising alternative to conventional HPLC techniques for the estimation of REM in the presence of LNZ and RIV. Different chromatographic conditions were adjusted to achieve good separation with high resolution, sharp symmetric peaks and satisfactory Rf values.
Method development and optimization
Different experimental parameters that affect the suggested TLC-densitometric technique such as mobile phase composition, saturation time, and scanning wavelengths, were adjusted.
First, a mixture of petroleum ether and ethyl acetate in different ratios was examined. LNZ and RIV separated well, but RIV and REM separated poorly.
Several solvent mixtures, such as ethyl acetate-methanol, ethyl acetate-ethanol, dichloromethane-methanol, dichloromethane-ethanol, dichloromethane-ethyl acetate and dichloromethane-acetone, were examined to enhance the separation.
Testing the ethyl acetate-methanol combination with different ratios, only LNZ and RIV were well resolved but RIV and REM were slightly resolved. Upon replacing methanol with ethanol, the mixture afforded merely resolved peaks with poor resolution.
Upon testing dichloromethane-methanol only REM was eluted, and the others were retained. While dichloromethane-ethyl acetate resulted in poor separation between LNZ and RIV. On the flip side, dichloromethane-ethanol has resulted in well resolved peaks however the REM peak has a tail. Thereby, acetic acid, formic acid, 25% ammonia solution, and triethylamine in different proportions were added to the tested mixture, however, poor resolution resulted. Eventually, a mixture of dichloromethane-acetone in different proportions was tested, yielding well-resolved sharp peaks with improved resolution. Therefore, mixture of dichloromethane-acetone in a ratio of 8.5:1.5 v/v was used throughout this approach.
Saturation times ranging from 15 to 45 min, were investigated since they had a considerable impact on chromatographic separation. With a saturation time of 30 min, satisfactory results were obtained. Different scanning wavelengths (235, 254, 280 nm) were tested, and 254 nm demonstrated the best sensitivity, yielding sharp, symmetrical peaks with minimal noise.
Finally, dichloromethane–acetone (8.5:1.5, v/v) mixture was chosen as the eluent at scanning wavelength 254 nm. The examined drugs were separated with acceptable Rf of 0.04, 0.23, 0.53, 0.72 for plasma, REM, LNZ and RIV respectively, (Figs. 2 and 3).
Method validation
ICH Q2(R1) recommendations have been implemented to evaluate the method’s validation criteria34, including:
Linearity and range
Under the above mentioned densitometric procedures, the regression plots were generated over the concentration range of 0.20–5.50, 0.20–4.50 and 0.10–3.00 μg/band for REM, LNZ and RIV, respectively. Good correlation coefficients were obtained, and linear regression equations were as follow:
where, y is the peak area at 254 nm, x is the concentration in µg/band, and R2 is the correlation coefficient. As illustrated by Table 1, the suggested technique has good linearity.
Accuracy
To evaluate the accuracy of the suggested method, the percentage recoveries of six concentrations of analytes within the linearity range were analyzed in triplicate. The obtained data has shown that the proposed method has a high degree of reliability and accuracy with small values of standard deviation (Table 2).
Intra- and inter-day precision
The proposed method’s intra- and inter-day precision was investigated. For intra-day precision, three different concentrations of each drug were measured in triplicate on the same day, and three successive days for inter-day precision. This was supported by the relative standard deviation (%RSD) values which were less than 2%, as shown in Table 3.
Detection and quantitation limits
The method’s sensitivity was evaluated by calculating detection and quantitation limits using the standard deviation of the intercept (σ) and the average slope (S); where LOD; (3.3*σ)/S and LOQ; (10*σ)/S, respectively. The resulting limits for REM, LNZ, and RIV are shown in Table 1 confirming that the proposed method has good sensitivity.
Robustness
Minor but intentional changes were made to the chromatographic technique parameters, and the percentage relative standard deviation (% RSD) was used to assess the robustness. Slight alteration was done in the proportions of the mobile phase system; dichloromethane, acetone (8.5:1.5 ± 0.10 mL), and saturation time (30 ± 5 min). The findings demonstrated the approaches’ reliability and robustness, demonstrating that the tested factors had no apparent impact (Table 4).
System suitability testing
System suitability tests showed good results compared to reference values for parameters like capacity factor, tailing factor, and resolution35,36. (Table 5).
Application to pharmaceutical formulations
The pharmaceutical formulations of REM, LNZ, and RIV were successfully estimated employing the suggested technique. The obtained percentage recoveries were satisfactory. Using the variance ratio F-test and the student’s t-test37, the results were compared to previously reported methods17,38,39. No significant differences were found, demonstrating the absence of excipient interference. The findings were acceptable, proving the proposed method’s high accuracy, Table 6.
Application to spiked human plasma
In human plasma, the new technique showed great sensitivity. The outcomes indicate that the method can accurately detect the investigated drug concentrations in human plasma without any interference, as shown in Fig. 3 and Table 7.
Green assessment of the proposed method
Green analysis relies on the use of green chemicals, limited usage of hazardous ones with no waste production and reduced energy consumption. Eco-Scale technique, the Green Analytical Procedure Index (GAPI) metric, and the Analytical GREEnness metric approach and Software were investigated to evaluate the suggested TLC-densitometric method’s greenness40,41,42. The green profiles for the proposed method using the mentioned metrics are shown in Table 8.
Analytical eco-scale assessment of the proposed method
Analytical Eco-scale is an easy-to-implement technique for quality control laboratory work. The analytical Eco Scale score is computed using the following formula: analytical Eco Scale score = 100-∑ penalty points. It is determined by allocating penalty points to each of the parameters of the outlined technique, including the amount of reagents, occupational hazards waste, and energy40. The analytical approach is recognized as an outstanding green analysis if the score is higher than 75. The designed TLC-densitometric technique has an Eco-Scale score of 91, as shown in Table 8a, indicating that the outlined analytical procedure is considered green.
GAPI assessment of the proposed method
A relatively recent method for gauging greenness is the green analytical process index (GAPI), created by Plotka-Wasylka41. It follows the entire process, from sample collection through waste processing. It presents 15 items to be examined using three degrees of color: green, yellow, or red, where red represents bad impact, yellow for intermediate, and green for safe and low environmental dangers. This allows for a thorough examination of each stage in the analytical procedure. The proposed technique fulfilled the majority of GAPI criteria with only 3 red regions. The first was caused by offline sample collection, the second by using a non-green solvent to precipitate plasma proteins, and the third by the absence of waste treatment possibilities. There are four yellow-colored regions corresponding to region 5, 10, 11 and 14. Region 5 is yellow as the method requires simple preparation such as filtration, while regions 10,11 are yellow because the solvents used have moderate health and environmental effects, according to the National Fire Protection Association (NFPA), and region 14 is yellow as the waste produced is less than 10 ml per sample. Overall, the results show that the suggested approach is environmentally friendly, as shown in Table 8b.
AGREE assessment of the proposed method
Recently, the AGREE assessment tool was reported. The assessment is carried out with ease, implementing user-friendly software with an automatically created graph, and an evaluation report42. AGREE provides a clock-shaped graph with a perimeter divided into 12 sections based on the 12 tenets of green analytical chemistry. To assess whether the analytical technique complies with the green analytical chemistry (GAC) concept, each division is assigned to a different color on a red-yellow-green scale. A color and a number representing the overall evaluation on a scale of 0 to 1 are located in the center of the AGREE graph. The proposed method scored 0.81 as demonstrated in Table 8c, which indicates the greenness characteristics of the developed method.
Conclusion
A simple, selective, and accurate chromatographic method has been developed for simultaneous determination of REM, LNZ and RIV in spiked human plasma. As multiple samples can be run simultaneously using minimal volumes of solvents, the TLC-densitometric approach saves time and lowers the cost of analysis. Moreover, the proposed method is eco-friendly with low impact on the environment. It has the benefits of being very selective, sensitive, and reproducible. It provides a fast and validated TLC analytical method for measuring remdesivir in the presence of linezolid and rivaroxaban for simple therapeutic medication monitoring and possible pharmacokinetic investigations.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- LNZ:
-
Linezolid
- REM:
-
Remdesivir
- RIV:
-
Rivaroxaban
- TLC:
-
Thin Layer Chromatography
- Rf :
-
Retardation factors
References
Sohrabi, C. et al. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 76, 71–76 (2020).
Magro, G. COVID-19: Review on latest available drugs and therapies against SARS-CoV-2 coagulation and inflammation cross-talking. Virus Res. 286, 198070 (2020).
Venkatasubbaiah, M., Reddy, P. D. & Satyanarayana, S. V. Literature-based review of the drugs used for the treatment of COVID-19. Curr. Med. Res. Pract. 10(3), 100–109 (2020).
Wouters, O. J. et al. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 397(10278), 1023–1034 (2021).
Al-Tannak, N. F., Novotny, L. & Alhunayan, A. Remdesivir—Bringing hope for COVID-19 treatment. Sci. Pharm. 88(2), 29 (2020).
Eastman, R. T. et al. Remdesivir: A review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent. Sci. 6(5), 672–683 (2020).
Gordon, C. J. et al. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 295(20), 6785–6797 (2020).
Lamb, Y. N. Remdesivir: First approval. Drugs 80, 1355–1363 (2020).
Alvarez, J. C., Moine, P., Etting, I., Annane, D. & Larabi, I. A. Quantification of plasma remdesivir and its metabolite GS-441524 using liquid chromatography coupled to tandem mass spectrometry. Application to a Covid-19 treated patient. Clin. Chem. Lab. Med. 58(9), 1461–1468 (2020).
Avataneo, V. et al. Development and validation of a UHPLC-MS/MS method for quantification of the prodrug Remdesivir and its metabolite GS-441524: A tool for clinical pharmacokinetics of SARS-CoV-2/COVID-19 and Ebola virus disease. J. Antimicrob. Chemother. 75(7), 1772–1777 (2020).
Nguyen, R. et al. Development and validation of a simple, selective, and sensitive LC-MS/MS assay for the quantification of Remdesivir in human plasma. J. Chromatogr. B 1171, 122641 (2021).
Pasupuleti, R. R., Tsai, P. C., Ponnusamy, V. K. & Pugazhendhi, A. Rapid determination of Remdesivir (SARS-CoV-2 drug) in human plasma for therapeutic drug monitoring in COVID-19-Patients. Process Biochem. 102, 150–156 (2021).
Raasi, K. M. Analytical method development and validation of Remdesivir in bulk and pharmaceutical dosage forms using reverse-phase-high performance liquid chromatography. BR Nahata Smriti Sansthan Int. J. Phram. Sci. Clin. Res. https://doi.org/10.22377/ijpscr.v1i2.35 (2021).
Xiao, D. et al. Validation of LC-MS/MS methods for determination of remdesivir and its metabolites GS-441524 and GS-704277 in acidified human plasma and their application in COVID-19 related clinical studies. Anal. Biochem. 617, 114118 (2021).
Abo-Gharam, A. H. & El-Kafrawy, D. S. Eco-friendly stability-indicating HPTLC micro-determination of the first FDA approved SARS-CoV-2 antiviral prodrug Remdesivir: Study of degradation kinetics and structural elucidation of the degradants using HPTLC-MS. Sustain. Chem. Pharm. 29, 100744 (2022).
Noureldeen, D. A., Boushra, J. M., Lashien, A. S., Hakiem, A. F. A. & Attia, T. Z. Novel environment friendly TLC-densitometric method for the determination of anti-coronavirus drugs Remdesivir and Favipiravir: Green assessment with application to pharmaceutical formulations and human plasma. Microchem. J. 174, 107101 (2022).
Bulduk, I. & Akbel, E. A comparative study of HPLC and UV spectrophotometric methods for Remdesivir quantification in pharmaceutical formulations. J. Taibah Univ. Sci. 15(1), 507–513 (2021).
Tkach VV, Kushnir MV, de Oliveira SC, Shevchenko IM, Odyntsova VM, Omelyanchik VM, et al. Theoretical description for anti-COVID-19 drug molnupiravir electrochemical determination over the poly-((1, 2, 4-triazole)-co-(squaraine dye)) composite with cobalt (III) oxyhydroxide. 13(1), 74 (2023) https://doi.org/10.33263/BRIAC131.074.
Elama, H. S., Zeid, A. M., Shalan, S. M., El-Shabrawy, Y. & Eid, M. I. Eco-friendly spectrophotometric methods for determination of Remdesivir and Favipiravir; the recently approved antivirals for COVID-19 treatment. Spectrochim. Acta A Mol. Biomol. Spectrosc. 287, 122070 (2023).
Elmansi, H., Ibrahim, A. E., Mikhail, I. E. & Belal, F. Green and sensitive spectrofluorimetric determination of Remdesivir, an FDA approved SARS-CoV-2 candidate antiviral; application in pharmaceutical dosage forms and spiked human plasma. Anal. Methods 13(23), 2596–2602 (2021).
Attia, T. Z., Boushra, J. M., Abdel Hakiem, A. F., Lashien, A. S. & Noureldeen, D. A. Spectrofluorimetric determination of the anti-Covid 19 agent, Remdesivir, in vials and spiked human plasma. Luminescence 37(7), 1192–1199 (2022).
El Sharkasy, M. E., Tolba, M. M., Belal, F., Walash, M. I. & Aboshabana, R. Simultaneous spectrofluorimetic determination of Remdesivir and Simeprevir in human plasma. Sci Rep. 12(1), 21980 (2022).
Wu, R. et al. An update on current therapeutic drugs treating COVID-19. Curr. Pharmacol. Rep. 6, 56–70 (2020).
Grau, S. et al. Evolution of antimicrobial consumption during the first wave of COVID-19 pandemic. Antibiotics 10(2), 132 (2021).
Moghadam, V. D., Momenimovahed, Z., Ghorbani, M. & Khodadadi, J. Linezolid a potential treatment for COVID-19 coinfections. Braz. J. Anesthesiol. 71, 198–198 (2021).
Morgon, N. H. et al. Potential activity of Linezolid against SARS-CoV-2 using electronic and molecular docking study. J. Mol. Model. 27, 1–11 (2021).
Arslan, Y. et al. The effectiveness of early anticoagulant treatment in Covid-19 patients. Phlebology 36(5), 384–391 (2021).
Spyropoulos, A. C. et al. Scientific and standardization committee communication: Clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 18(8), 1859–1865 (2020).
Ibrahim, A. E., Deeb, S. E., Abdelhalim, E. M., Al-Harrasi, A. & Sayed, R. A. Green stability indicating organic solvent-free HPLC determination of Remdesivir in substances and pharmaceutical dosage forms. Separations 8(12), 243 (2021).
Cazorla-Reyes, R., Romero-González, R., Frenich, A. G., Rodríguez Maresca, M. A. & Martínez Vidal, J. L. Simultaneous analysis of antibiotics in biological samples by ultra high performance liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 15(89), 203–212 (2014).
Lee, K. et al. Multiplex assay of second-line anti-tuberculosis drugs in dried blood spots using ultra-performance liquid chromatography-tandem mass spectrometry. Ann. Lab. Med. 36(5), 489 (2016).
Çelebier, M., Reçber, T., Koçak, E., Altınöz, S. & Kır, S. Determination of Rivaroxaban in human plasma by solid-phase extraction-high performance liquid Chromatography. J. Chromatogr. Sci. 54(2), 216–220 (2016).
Çelebier, M., Reçber, T., Koçak, E. & Altinöz, S. RP-HPLC method development and validation for estimation of Rivaroxaban in pharmaceutical dosage forms. Braz. J. Pharm. Sci. 49, 359–366 (2013).
ICH Harmonized Tripartite Guideline, V.o.A.P.T.a.M., Q2(R1), Current Step 4 Version, Parent Guidelines dated 27 October 1994, complementary guidelines on Methodology Dated 6 November 1996, Incorporated in November 2005.
The United States pharmacopeia [USP online]. (The National Formulary 27).
Variyar, P. S., Chatterjee, S. & Sharma, A. Fundamentals and theory of HPTLC-based separation. In High-Perform Thin-Layer Chromatogr HPTLC (ed. Srivastava, M.) (Springer, 2011).
Miller, J. & Miller, J. C. Statistics and Chemometrics for Analytical Chemistry (Pearson Education, 2018).
Patel, S. A., Patel, P. U., Patel, N. J., Patel, M. M. & Bangoriya, U. V. Determination of linezolid in pharmaceutical dosage forms by liquid chromatography and ultraviolet spectroscopy. J. AOAC Int. 90(5), 1272–1277 (2007).
Bhavyasri, K., Dhanalakshmi, C. & Sumakanth, M. Development and validation of ultra violet-visible spectrophotometric method for estimation of Rivaroxaban in spiked human plasma. J. Pharm. Sci. Res. 12(9), 1215–1219 (2020).
Gałuszka, A., Migaszewski, Z. M., Konieczka, P. & Namieśnik, J. Analytical eco-scale for assessing the greenness of analytical procedures. TrAC Trends Anal. Chem. 1(37), 61–72 (2012).
Płotka-Wasylka, J. A new tool for the evaluation of the analytical procedure: Green analytical procedure index. Talanta 1(181), 204–209 (2018).
Pena-Pereira, F., Wojnowski, W. & Tobiszewski, M. AGREE—Analytical GREEnness metric approach and software. Anal. Chem. 92(14), 10076–10082 (2020).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Ekram A. Ghozzy carried out the laboratory work, participated in data analysis, participated in the design of the study and drafted the manuscript. Nahed M.El-Enany conceived of the study, designed the study and coordinated the study. Manar M. Tolba participated in data analysis, in the design of the study and drafted the manuscript. Samah Abo El Abass carried out the statistical analyses, conceived of the study, designed the study and submitted the manuscript. All authors gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghozzy, E.A., El-Enany, N.M., Tolba, M.M. et al. An eco-friendly and cost-effective HPTLC method for quantification of COVID-19 antiviral drug and co-administered medications in spiked human plasma. Sci Rep 14, 10025 (2024). https://doi.org/10.1038/s41598-024-56923-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-56923-4
Keywords
This article is cited by
-
Green High-Performance Thin-Layer Chromatography: A Step Towards Eco-Friendly Analysis
Chromatographia (2025)