Abstract
Background
Frailty is associated with mortality in older adults hospitalized with COVID-19, yet few studies have quantified healthcare utilization and spending following COVID-19 hospitalization.
Objective
To evaluate whether survival and follow-up healthcare utilization and expenditures varied as a function of claims-based frailty status for older adults hospitalized with COVID-19.
Design
Retrospective cohort study
Participants
136 patients aged 65 and older enrolled in an Accountable Care Organization (ACO) risk contract at an academic medical center and hospitalized for COVID-19 between March 11, 2020 - June 3, 2020
Measurements
We linked a COVID-19 Registry with administrative claims data to quantify a frailty index and its relationship to mortality, healthcare utilization, and expenditures over 6 months following hospital discharge. Kaplan Meier curves and Cox Proportional Hazards models were used to evaluate survival by frailty. Kruskal-Wallis tests were used to compare utilization. A generalized linear model with a gamma distribution was used to evaluate differences in monthly Medicare expenditures.
Results
Much of the cohort was classified as moderate to severely frail (65.4%), 24.3% mildly frail, and 10.3% robust or pre-frail. Overall, 27.2% (n=37) of the cohort died (n=26 during hospitalization, n=11 after discharge) and survival did not significantly differ by frailty. Among survivors, inpatient hospitalizations during the 6-month follow-up period varied significantly by frailty (p=0.02). Mean cost over follow-up was $856.37 for the mild and $4914.16 for the moderate to severe frailty group, and monthly expenditures increased with higher frailty classification (p <.001).
Conclusions
In this cohort, claims-based frailty was not significantly associated with survival but was associated with follow-up hospitalizations and Medicare expenditures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The coronavirus SARS-CoV-2 pandemic continues to result in millions of confirmed cases of COVID-19. Although vaccinations successfully decreased the number of older adults hospitalized with COVID-19 in early to mid-2021, waning immunity and the rise of new variants in recent months has sustained concern regarding hospitalizations and mortality risk in this population.
Many patient characteristics, such as multimorbidity, functional status, and frailty influence outcomes for older adults hospitalized with COVID-19 (1, 2). Frailty, in particular, has been an emphasized area of study for this population (3). This is unsurprising, given robust evidence of the role of frailty in adverse outcomes among older adults (4). In fact, the National Institute for Health and Care Excellence (NICE) in the United Kingdom and the Belgian Society of Intensive Care Medicine promote the use of frailty assessment in care planning for older adults with COVID-19 (5, 6). Growing evidence demonstrates increased risk of mortality among older adults hospitalized for COVID-19 who are frail, assessed primarily by the Clinical Frailty Scale (CFS) (3), which is widely implemented in Europe and Canada. Thus, most frailty research in this area has come from these two geographic regions with scant evidence coming from the US.
This is likely due, in part, to slow implementation of standardized frailty assessment in the US, given lack of agreement on which measure to use across clinical settings and patient populations (7, 8). Despite this lack of consensus, frailty indexes based on the cumulative deficit model of frailty in aging have been increasingly used in research to evaluate risk of poor outcomes (9). These indices generate a frailty estimate as a ratio (0 to 1) using 30–70 different clinical factors (signs, symptoms, laboratory values, disabilities, etc.) often aligned with measures in comprehensive geriatric assessment. Although frailty indexes have been associated with mortality in several populations (10, 11), their association with mortality and other outcomes for older adults hospitalized with COVID-19 in the US remains unknown.
The goal of this study was to leverage a claims-based frailty index (CFI) to investigate the association between frailty and outcomes among a cohort of older adults hospitalized for COVID-19 in the US using an existing COVID-19 patient registry from an academic medical center linked with administrative claims data. Specifically, we characterize demographic, clinical, and hospitalization characteristics of this population by frailty status using a validated CFI and examine differences in mortality, healthcare utilization, and Medicare expenditures post-hospitalization.
Methods
Study Sample
We used data from the Massachusetts General Hospital (MGH) COVID-19 Data Registry to identify patients aged 65 years and older who were hospitalized for PCR confirmed SARS-CoV-2 between March 11, 2020 – June 3, 2020 (12). The registry was developed using coded data extraction from the Mass General Brigham Enterprise Data Warehouse and manual chart review of electronic health records. Of the 1,391 patients in the registry, 549 were aged 65 and older. We then identified a subset of older adults who belonged to Mass General Brigham Medicare Accountable Care Organization (ACO) risk contract, which enabled us to use Medicare claims to characterize frailty, utilization, and expenditures. A total of 158 patients were enrolled in the ACO risk contract for at least one month in the year prior to their COVID admission. To ensure validity of frailty classification, we further excluded patients with < 9 months of continuous ACO enrollment in the year prior to hospitalization for a final study cohort of 136 patients (12-months n=122; 10-months n=3; 9-months n=11, Appendix Figure 1). Medicare claims were also used to examine healthcare utilization and costs following hospitalization; patients who died during hospitalization or had less than 1 month of ACO alignment were excluded from the follow-up utilization and cost analytic cohort (n=96). This study received exempt approval from our Institutional Review Board.
Frailty Assessment
We used a CFI algorithm to compute frailty scores for each patient using administrative claims in the 12 months prior to COVID hospitalization (13). The CFI employs a cumulative deficit approach to estimating frailty status using ICD-10 diagnosis codes, current procedural terminology (CPT) codes, and the healthcare common procedure coding system (HCPCS) from Medicare administrative data files over a 12 month look back period (13, 14). Scores range from 0 to 1, with higher scores representing higher levels of frailty. Open-source code for calculating the CFI is available at https://dataverse.harvard.edu. Appendix Table 1 provides a listing of administrative code categories included in the CFI. We classified patients as robust/ pre-frail (CFI <0.25), mildly frail (CFI 0.25 to <0.35) and moderate to severely frail (CFI ≥0.35) (15).
Outcomes
Electronic health records identified date of death and date of last known follow-up in our health system in the cohort for survival analysis. The time frame for survival analysis started at hospital admission and continued for 6 months. Medicare claims characterized utilization per member per month (PMPM) and expenditures among survivors of the COVID-19 hospitalization who remained enrolled in the ACO during follow-up. Utilization characteristics include ED visits defined as treat-and-release (i.e., not resulting in an observation or inpatient stay) and observation visits that did not result in an inpatient admission. Inpatient admissions include all hospital stays (Medical, Surgical, and Psychiatric), excluding skilled nursing stays and inpatient rehabilitation. Total Medicare expenditures (TME) represent the sum of all medical costs (excluding prescription drug costs) billed to Medicare each month over the 6 months following discharge.
Demographic and Clinical Characteristics
We describe the following demographic characteristics at hospital admission: age, sex, race/ethnicity, residence in a nursing home (NH) or assisted living facility (ALF), living alone, and being dual eligible for Medicare and Medicaid. Healthcare utilization and TME in the year prior to COVID hospitalization were characterized using Medicare claims. COVID registry data was used to identify clinical characteristics (e.g., chronic conditions, vital signs, use of supplemental oxygen, and symptoms at hospital admission), admission to the intensive care unit, and hospital length of stay.
Analysis
We used descriptive statistics to characterize demographic and clinical characteristics for the full sample and by frailty status. Continuous data are presented using means/standard deviations and median/interquartile range. Categorical data are presented using proportions. We constructed a KaplanMeier curve to examine survival in the cohort by frailty status. We then ran Cox proportional-hazards models to evaluate the association between frailty and mortality when adjusted for factors independently associated with survival (residing in a NH/ALF and being dual eligible). To evaluate potential bias in survival analyses, we compared results to survival analyses in the subgroup of patients who had the full 12 months of ACO enrollment prior to admission (n=122).
Given the non-normal distribution of data, Kruskal-Wallis rank sum tests were used to evaluate utilization and a generalized linear model with gamma distribution and log-link function was used to evaluate differences in monthly Medicare expenditures. All analyses were performed using SAS version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Sample Characteristics
Much of the sample was classified as moderate to severely frail (65.4%), 24.3% mildly frail, and 10.3% robust or pre-frail (Table 1). A large proportion of the robust/pre-frail group resided in a NH or ALF (42.9%), followed by moderate to severely frail (39.3%), and mildly frail (9.1%). Pre-COVID healthcare utilization and expenditures increased progressively with higher ordered frailty classification.
At hospital admission, the average body mass index was 28.8 kg/m2 and patients presented with a wide array of chronic conditions (Appendix Table 2). The most frequently documented chronic conditions at hospital admission were hypertension, dyslipidemia, diabetes, and coronary artery disease. A total of 41.4% of the cohort required supplemental oxygen on admission and the most commonly occurring symptoms were cough (62.5%), fever (48.5%) and shortness of breath (39.7%). Approximately ¼ of patients were admitted to the intensive care unit, a rate that was consistent across frailty groups.
Survival by Frailty Status
Overall, 26 patients died during hospitalization. Of the 11 patients who died after hospital discharge, 9 were classified as moderate to severely frail. Rates of survival were not statistically significant between frailty groups (log rank test, p=0.21; Figure 1), although the probability of survival was lowest among those classified as robust/pre-frail, followed by mild frailty and moderate to severe frailty. Being a NH/ ALF resident was associated with poorer survival (log rank test, p=0.003) while being dual eligible was associated with better survival (log rank test, p=0.004). In the adjusted Cox proportional-hazards model, frailty remained unassociated with survival (Wald χ2=3.8, p=0.15) while NH/ALF residency remained strongly associated with poorer survival (Hazard Ratio (HR): 3.0 (95% Confidence Interval (CI): 1.5, 6.2)), and dual-eligibility with better survival (HR: 0.21 (95% CI: 0.1, 0.6)). In sensitivity analyses with patients with 12 months of ACO enrollment, frailty was significantly associated with survival (log rank test, p=0.02; Appendix Figure 2), due to high mortality during hospitalization (n=5, 62.5%) in a smaller robust/pre-frail group (n=8).
Healthcare Utilization and Expenditures
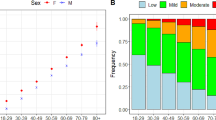
Healthcare utilization and TME varied over the 6 months following COVID hospital discharge between frailty groups (Appendix Table 3). Inpatient stays were significantly higher among the moderate to severely frail (χ2=7.48, p=0.02). Mean TME PMPM for the entire 6-month period among survivors was $4,116.22. Although monthly TME were highest for the moderate to severely frail group, expenditures among the mild frailty group declined over time with no billed costs among the robust/pre-frail (Figure 2). Differences in monthly expenditures between frailty groups were statistically significant (F-value=292.3, p <.0001; Appendix Table 4). For the robust/pre-frail group, zero cost remained stable across the 6 months, whereas the mean cost over follow-up was $856.37 for the mild and $4914.16 for the moderate to severe frailty group. Observed differences in average monthly costs were significantly higher for the mildly frail (p<.0001) and moderate to severely frail groups (p<.0001) when compared to the robust/ pre-frail.
Discussion
In a cohort of Medicare ACO risk contract members aged 65 years and older who were hospitalized during the first surge of the pandemic, claims-based frailty was not significantly associated with overall survival. Residing in a NH or ALF was significantly associated with poorer survival while being dually eligible was associated with improved survival. Among surviving ACO members, inpatient stays and Medicare expenditures were significantly higher among older adults classified as frail, with persistently high costs noted over follow-up among individuals in the moderate to severe frailty group.
Surprisingly, the worst survival probabilities were noted for individuals classified as robust/pre-frail, followed by mild and moderate to severe frailty. Additionally, while hospital deaths were higher in the robust/pre-frail group, mortality after hospital discharge was highest in the moderate to severe frailty group. Potential explanations include higher median age (83.2 years) and larger proportion of individuals who resided in NH or ALF (42.9%) in the robust/pre-frail group, two characteristics associated with higher mortality (16, 17). Additionally, nearly 50% of patients who were dual-eligible were younger than 75 years of age, which may partially explain better survival. Although few COVID-19 studies use frailty indexes to investigate frailty (18, 19), in a comparison of the CFS and a frailty index in older adults, the frailty index was not predictive of critical illness (19). Potential lack of predictive validity for critical illness and mortality may be due to the methods used to derive administrative data-based frailty indexes, which rely on diagnosis and procedure rather than clinical assessment. Although the CFI provides a useful method for stratifying populations by characterizing those with more comorbid conditions and morbidity-related deficits, it has the potential to overestimate frailty status due to reimbursement-driven accumulations of provider billed diagnosis codes in claims data (11).
In contrast to results from our survival models, post-hospitalization inpatient stays and expenditures varied by frailty status, with no utilization or costs noted among the robust/pre-frail, moderate rates among the mildly frail, and persistently high rates among the moderate to severe frailty group. The observed association between CFI, utilization, and expenditures in our cohort aligns with prior validation of the measure (13) and a recent study that demonstrated improved prediction of future costs among Medicare beneficiaries using the CFI (20). Taken together, these findings suggest that leveraging claims data to estimate CFI-defined frailty groups among patients enrolled in ACOs may be a useful method for identifying potentially high-cost patients.
Limitations
This cohort study is the first to evaluate differences in survival and post-COVID hospitalization utilization and costs among older adults at an academic medical center in the US, yet findings should be interpreted with caution. Our sample was derived using a COVID-19 registry from a single academic medical center, which may decrease the generalizability of results. Nearly 1/3 of our sample lived in a NH or ALF, a population with higher illness burden and frailty prevalence, which also limits generalizability. To accurately characterize subsequent healthcare utilization and expenditures after COVID-19 hospitalization, we used a subgroup of patients from the registry that were enrolled in our ACO risk contract, which limited our sample size. As a result, the study may have been underpowered to detect a true difference in survival based on frailty classification. Additionally, we used a claims-based cumulative-deficit approach to frailty classification, which uses 12-months of retrospective claims data and may not capture symptom severity or management. We were unable to compare performance of the CFI to clinical assessment of physical frailty, functional status, and other relevant characteristics that may have been associated with mortality, utilization, and costs.
Conclusion
In conclusion, claims-based frailty was not significantly associated with survival among older adults hospitalized with COVID-19 but was associated with increased post-hospitalization inpatient stays and expenditures. As COVID-19 becomes endemic, claims-based frailty indices may serve as a useful indicator of risk for future utilization.
References
Farrell TW, Ferrante LE, Brown T, et al. AGS Position Statement: Resource Allocation Strategies and Age-Related Considerations in the COVID-19 Era and Beyond. J Am Geriatr Soc 2020;68:1136–1142
Cesari M, Proietti M. COVID-19 in Italy: Ageism and Decision Making in a Pandemic. J Am Med Dir Assoc 2020;21:576–577
Dumitrascu F, Branje KE, Hladkowicz ES, Lalu M, McIsaac DI. Association of frailty with outcomes in individuals with COVID-19: A living review and meta-analysis. J Am Geriatr Soc 2021;69:2419–2429
Vermeiren S, Vella-Azzopardi R, Beckwée D, et al. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J Am Med Dir Assoc 2016;17:1163.e1–1163.e17; DOI: https://doi.org/10.1016/j.jamda.2016.09.010
COVID-19 rapid guideline: critical care in adults. London: National Institute for Health and Care Excellence (NICE); 2021 Feb 12. PMID: 33497153.
Voorde PV de, Monsieurs K, Ranier W, et al. Ethical Principles and guidance with regard to ethical decisions in pre-hospital and emergency medicine in Belgium during the COVID-19 pandemic. A joint statement of the Belgian Society of Emergency and Disaster Medicine and the Belgian Resuscitation Council. 2020
Bandeen-Roche K, Gross AL, Varadhan R, et al. Principles and Issues for Physical Frailty Measurement and Its Clinical Application. Journals Gerontology Ser 2019;75:1107–1112
Walston J, Bandeen-Roche K, Buta B, et al. Moving Frailty Toward Clinical Practice: NIA Intramural Frailty Science Symposium Summary. J Am Geriatr Soc 2019;67:1559–1564
Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016;26:53–61
Rockwood K, Mitnitski A. Frailty in Relation to the Accumulation of Deficits. Journals Gerontology Ser 2007;62:722–727
Kim DH. Measuring Frailty in Health Care Databases for Clinical Care and Research. Ann Geriatric Medicine Res 2020;24:62–74
Bassett IV, Triant VA, Bunda BA, et al. Massachusetts general hospital Covid-19 registry reveals two distinct populations of hospitalized patients by race and ethnicity. Plos One 2020;15:e0244270; DOI: https://doi.org/10.1371/journal.pone.0244270
Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. Journals Gerontology Ser 2018;73:980–987
Kim DH, Patorno E, Pawar A, Lee H, Schneeweiss S, Glynn RJ. Measuring Frailty in Administrative Claims Data: Comparative Performance of Four Claims-Based Frailty Measures in the United States Medicare Data. Journals Gerontology Ser 2019;75:1120–1125
Kim DH, Glynn RJ, Avorn J, et al. Validation of a Claims-Based Frailty Index Against Physical Performance and Adverse Health Outcomes in the Health and Retirement Study. Journals Gerontology Ser 2018;74:1271–1276
Bonanad C, García-Blas S, Tarazona-Santabalbina F, et al. The Effect of Age on Mortality in Patients With COVID-19: A Meta-Analysis With 611,583 Subjects. J Am Med Dir Assoc 2020;21:915–918
Panagiotou OA, Kosar CM, White EM, et al. Risk Factors Associated With All-Cause 30-Day Mortality in Nursing Home Residents With COVID-19. Jama Intern Med 2021;181:439–448
Shi SM, Bakaev I, Chen H, Travison TG, Berry SD. Risk Factors, Presentation, and Course of Coronavirus Disease 2019 in a Large, Academic Long-Term Care Facility. J Am Med Dir Assoc 2020;21:1378–1383.e1; DOI: https://doi.org/10.1016/j.jamda.2020.08.027
Lim JP, Low KYH, Lin NJJ, et al. Predictors for development of critical illness amongst older adults with COVID-19: Beyond age to age-associated factors. Arch Gerontol Geriat 2021;94:104331–104331; DOI: https://doi.org/10.1016/j.archger.2020.104331
Johnston KJ, Wen H, Maddox KEJ. Relationship of a Claims-Based Frailty Index to Annualized Medicare Costs: A Cohort Study. Ann Intern Med 2020;172:533
Acknowledgements
Support for the MGH COVID-19 Registry was provided by the MGH Division of Clinical Research and the Department of Medicine.
Funding
Funding: none.
Author information
Authors and Affiliations
Contributions
Author Contributions: TK, CR, CV, and DK contributed to study concept and design. MF, JD, MS, and KL assisted with acquisition of data. TK analyzed data; MF, DK, JO, CV, and CR provided feedback on data analyses and interpretation of results. TK drafted the manuscript and all authors contributed critical feedback and major revisions during manuscript development. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest: Dr. Kim receives personal consulting fees from Alosa Health and VillageMD.
Ethical standards: This study was approved by the MGH Institutional Review Board, Protocol # 2020P001936. The MGH IRB approved an informed consent waiver for this study. All methods were performed in accordance with the relevant guidelines and regulations.
Supplemental Data
Rights and permissions
About this article
Cite this article
Keeney, T., Flom, M., Ding, J. et al. Using a Claims-Based Frailty Index to Investigate Frailty, Survival, and Healthcare Expenditures among Older Adults Hospitalized for COVID-19 at an Academic Medical Center. J Frailty Aging 12, 150–154 (2023). https://doi.org/10.14283/jfa.2023.15
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jfa.2023.15