Abstract
The aim of the study was to conduct of relationship of acute-phase proteins (APPs) with the severity of COVID-19 defined by National Institutes of Health and according to the criteria of MEWS scale, with the presence of a cytokine storm, oxygen therapy and patient survival. We enrolled 96 patients with COVID-19 and 30 healthy people. The samples were taken on the day of admission and after 9 days on average. Not only commonly used APPs such as CRP, procalcitonin and ferritin and also rarely assayed proteins such as transferrin, haptoglobin, α1-acid glycoprotein and α1-antitrypsin, were tested in the study. The levels of APPs depends on the severity of COVID-19 disease, on the presence of cytokine storm and used oxygen therapy. The greatest APPs changes occurred in the most advanced form of the disease, with the presence of a cytokine storm and the most intense oxygen therapy. The results obtained from MEWS scale were not consistent with National Institutes of Health scores. Studies in the second samples showed the quenching of the acute phase reactions and the effectiveness of oxygen therapy. Only two of the examined APPs i.e. procalcitonin and transferrin, differed between surviving and non-surviving patients, and these two predispose to the role of prognostic factors in Covid-19. In conclusion, the concentration of not all acute-phase proteins depends on the severity of COVID-19 disease, presence of cytokine storm, the used of oxygen therapy and only some of them (procalcitonin and transferrin) are related to the survival outcomes. Of the newly tested acute-phase proteins, only transferrin shows significance as a marker of disease severity and mortality in COVID-19 disease.
Similar content being viewed by others
Introduction
Infection by SARS-CoV-2 coronavirus induces human immune system to inflammatory response which in turn stimulates the liver to synthesis acute-proteins (APPs)1,2. They exhibit significant changes in the serum concentration during inflammation. If the concentration of proteins increases these are called as positive acute-phase proteins but if decreases these are named negative acute-phase proteins3,4,5. C-reactive protein (CRP), α1-acid glycoprotein (AGP), α1-antitrypsin (AAT), ferritin (FER), procalcitonin (PCT), haptoglobin (HAP) and fibrinogen (FIB) are positive APPs and albumin, prealbumin, transferrin (TRF), cortisol-binding globulin and retinol-binding protein are negative APPs.
There is little data on the relationship between APPs and the course of COVID-19 disease, with the exception of CRP, PCT and FER6,7,8,9,10. Some of them indicate the importance of acute phase proteins as prognostic indicators of disease severity and mortality6,9,10,11,12,13. In the one study, co-called composite poor outcomes including severity, mortality, acute respiratory distress syndrome and the need for care in an intensive care unit, were associated with an elevated CRP and PCT9. An another study showed the higher proportion of patients with increased level of CRP and PCT in severe disease than in the mild, and that CRP could be used as independent factor to predict the severity of COVID-1910. The study enrolling 125 patients with COVID-19 demonstrated the positive correlation of FER and CRP with the progression of the illness12, and in the another studies PCT, FER and CRP were significantly higher in severe than in the mild disease13. Therefore, in this work we want to conduct a comprehensive analysis of factors that may have a potential relationship with the course of the disease. They will address the severity of the disease, including the MEWS score, the presence of a cytokine storm, oxygen therapy, and patient survival. Although some of the APPs have already been studied, in this work we propose a comprehensive comparison of those that are commonly assayed (CRP, PCT and FER) with those that not yet been studied, including HAP, TRF, AGP and AAT.
Materials and methods
Patients
The study was approved by the Bioethical Committee at the Medical University of Bialystok (Approval No. APK. 002.174.2023). All procedures were in accordance with the Declaration of Helsinki (2020/340). Healthy and sick patients provided informed consent to participate in the studies.
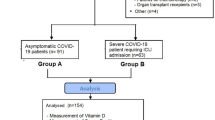
The study included 96 patients with COVID-19 (56 men in median age 62 years; range: 22–85, 40 women in median age 64 years; range: 23–89) and 30 healthy people. All patients were newly diagnosed and untreated during the time of admission to the Department of Gastroenterology, Hepatology and Internal Diseases with the Center for Diagnostics and Endoscopic Treatment in Bialystok (Poland). Blood samples were drawn twice, after admission (first sample –B1) and after 9 days on average (range: 4–11 days) (second sample –B2). The second collection took place before the patient was discharged home or transferred to the intensive care unit.
Diagnosis was confirmed testing for SARS-CoV-2 RNA by qualitative real-time reverse transcription polymerase chain reaction (RT-PCR) (SARS-Cov-2 Triplex PCR kit from Astra Biotech GmbH (Berlin, Germany) with thermocycler Azure Cielo 6 (Azure Biosystems, Dublin, USA) after the qualitative detection of SARS-CoV-2 antigen (Ag) in human nasal swab specimens with PanbioPM COVID-19 Ag rapid test device (Abbott Rapid Diagnostics Jena GmbH, Jena, Germany). 13 of 96 patients did not survive (mortality – 13.5%) and 9 were transferred to the intensive care unit (9.4%). A moderate condition of disease (fever, cough, anosmia and lack of dyspnea, lower respiratory tract involvement, with oxygen saturation > 94% and minor radiographic changes) was diagnosed in 63 patients, severe (oxygen saturation decreases < 94% at respiratory rate > 30/min, radiologically pulmonary infiltrates > 50% with respiratory failure) in 14 patients, and critical (multi-organ dysfunction with respiratory failure) in 19 patients (according to National Institutes of Health, 2020). In 76 patients were used oxygen therapies: low-flow nasal cannula oxygen (up to 18 l/min at an oxygen concentration of 25–35%) in 43 patients, high-flow nasal cannula oxygen (oxygen flow 20–60 l/min, concentration set depending on oxygen saturation 40–100%) in 23 patients and respiratory therapy in 10 patients. Cytokine storm was diagnosed in 26 patients (when interleukin-6 [IL-6] level was above 100 pg/ml). The control group consisted of 30 healthy subjects (15 men and 15 women), aged 21–84. A comparison of laboratory parameters among patients is provided in Table 1.
Analysis
APPs were determined on Cobas 6000 analyzer, C 501 module (Hitachi, Tokyo, Japan), hematology test on XN-1000 analyzer (Sysmex Corporation, Singapore), coagulometric tests (INR, FIB and PT) on ACL TOP 300 CTS analyzer (Instrumentation Laboratory, Werfen Company, Bedford, USA), blood gas and co-oximetry on ABL 90 FLEX PLUS analyzer (Radiometer Medical ApS, Brønshøj, Denmark), PCT and IL-6 on COBAS e-411 (Roche Diagnostics International Ltd, Rotkreuz, Switzerland).
Statistical analysis
Results were expressed as median and ranges. The differences between tested and control groups were evaluated by Mann–Whitney U-test. The effect of the disease severity, oxygen therapy and modifited early worning score (MEWS) on the concentration of isoforms was tested by the ANOVA rank Kruskal–Wallis test. The Spearman’s rank correlation coefficient was used to assess the correlation between TRF isoforms and laboratory tests. We considered P values < 0.05 as statistically significant.
Institutional review board statement
This study was approved by the Bioethical Committee at Medical University of Bialystok (Approval APK. 002.174.2023).
Results
Acute-phase proteins in COVID-19 patients and the control group
The concentrations of almost all APPs, except AAT, were significantly different in the first sample (B1) and in the second sample (B2) compared to the control group (Fig. 1). AAT concentrations differed only in sample B1. The direction of changes in APPs compared to the control group is an increase in concentration, with the exception of TRF, whose concentrations decreased in the tested group. The concentrations of APPs in sample B2 were lower than in sample B1, with the exception of PCT and TRF. The FER concentration in sample B2 was higher than in sample B1.
Acute-phase proteins according to the severity of the disease
The concentrations of CRP, FER, PCT and TRF showed differences depending on the severity of the disease according to NIH guidelines in the first sample (moderate, severe and critical state) (Fig. 2). As a general rule, there is a difference in the concentration of APPs between moderate and severe and critical condition. The concentrations of CRP, FER and PCT were significantly higher in the critical condition of the disease compared to the moderate, and the TRF concentration was significantly lower in the critical state of the disease than in the moderate. Additionally, for CRP, PCT and FER there was a difference in concentration between the moderate and severe condition of the disease, the concentrations in the severe state were significantly higher than in the moderate. In sample B2, the only difference observed was the difference in CRP concentration between critical (median: 310 mg/l; range: 3.1–452.0) and moderate condition (median: 18.3 mg/l, range: 0.8–273.9)(P < 0.001). The remaining APPs did not show any differences depending on the severity of the disease. The statistics for these tests are as follows (HAP: H = 3.644 and P = 0.303; AGP: H = 2.977 and P = 0.395; AAT: H = 5.762 and P = 0.124 in Kruskal–Wallis test).
Acute-phase proteins depending on the MEWS scale
Among the acute-phase proteins, only CRP and PCT concentrations showed an association with the early warning score (MEWS) (Table 2).
Post-hoc analysis showed statistically significant difference in CRP concentration (p = 0.042) between patients who obtained “O” points (Me = 10.70 mg/l, range: 10.60–14.70) and patients who obtained “6” points (Me = 224.5 mg/l, range: 55.60–452). In the case of PCT, a significant difference in the concentration (p = 0.040) occurred between patients who obtained “1” point (Me = 0.085 ng/ml, range: 0.02–6.02) and those who obtained “3” points (Me:0.29 ng/ml, range: 0.08–6.02).
Acute-phase proteins depending on the presence of cytokine storm
Among the APPs, only HAP and AGP did not show statistically significant differences depending on the presence of cytokine storm (Fig. 3). Among those that differed significantly, we noted a significant increase in the concentration of APPs in patients with cytokine storm, with the exception of TRF, the concentration of which was higher in patients without cytokine storm. Median IL-6 concentration in patients with cytokine storm was more than 6 times higher than in patients without cytokine storm (194 pg/ml, range: 1.9–127 vs 30.4 ng/ml, range: 96.6–990). For PCT it was a 4.6-fold increase, for CRP—almost fourfold, for FER—more than threefold, for AAT—an increase by 24%, while for TRF a decrease by 30%. The concentration of IL-6 correlated with all tested glycoproteins (Table 3).
Acute-phase proteins depending on the oxygen therapy
The analysis showed the differences in the concentrations of APPs depending on the decision to use oxygen therapy (sample B1) without AAT (Kruskal–Wallis ANOVA rank test: H = 2.334; P = 0.506) (Fig. 4). The main change captured was the difference in APPs concentrations between high-flow oxygen therapy and respiratory therapy and no oxygen therapy. This changes were recorded for CRP, FER, PCT, TRF and AGP. For FER and AGP, the differences between low oxygen flow (N) and no support were additionally demonstrated. For HP, the only difference in the concentration occurred between respiratory therapy and no oxygen respiratory support.
After oxygen therapy (sample B2), the concentrations of APPs did not show any changes depending on the type of therapy, with the exception of CRP, which was significantly higher in patients undergoing respiratotherapy (median: 98.03 mg/l; range: 43.90–138.80) than in those without oxygen therapy (median: 7.10 mg/l; range: 0.80–241.0) (P = 0.032).
Acute-phase proteins depending on the mortality
In non-surviving patients, higher PCT concentrations and lower TRF concentrations than that in surviving were obtained (sample B2) (Tables 4 and 5).
Discussion
It is not surprising that the concentrations of positive APPs increased in COVID-19 patients and TRF concentrations decreased. Of course, our results are not innovative at this point, but are in agreement with the results of other authors11,12,13. In each case, except for AAT, also in the sample from the second collection, the concentration was changed compared to healthy people. There were also significant differences in APPs concentrations between second and first sample, except for PCT and TRF. The concentrations in the second sample were lower than in the first sample. This is an obvious effect of quenching the acute-phase reaction.
This lack of change in TRF concentration in the second sample is a natural consequence of its longest half-life (8–10 days) compared to other glycoproteins14. The question arises how to explain this lack of change in PCT concentration, the half-life of which is one of the shortest (25–30 h)15? The persistently high level of PCT could be thought to the additional production of PCT from other disease-affected body tissues (liver, kidney, spleen and pancreas) in response to cytokine storm16.
This response is secondary (delayed in time) to the impact of the cytokine storm on the respiratory system, as evidenced by higher levels of ALT and GGT in the second sample than that in the first.
Taking into account the severity of disease we observed an increased level of positive APPs such as CRP, PCT and FER, and decreased level of TRF, with the increase in the severity of the disease. The significant differences were noted between moderate and severe, and moderate and critical condition. These results are in complete agreement with the results of other authors9,10.
The MEWS scale is used to identify patients who are at risk of clinical deterioration and who require a high level of care17. A score of ≥ 5 or a single physiological parameter score of 3 was accepted as a critical for significant increase in relative risk and higher level of care18. In this study, only two APPs, i.e. CRP and PCT, showed a relationship with the clinical stage of the disease. Only 4.4% of patients had a score ≥ 5 on the MEWS scale, while as many as 18.75% of patients were classified as critical on the disease severity scale. This means that the results obtained from MEWS scale did not match the scale of the disease's severity. Comparing both of these disease severity scales, it can be seen that their convergence concerns only oxygen saturation. The discrepancy between the results of MEWS scale and the severity of the disease results from the fact that the variables incorporated in the MEWS scale are a clinical consequence of biochemical changes in the body i.e. cytokine storm and inflammatory reaction, which primarily result in biochemical changes (including APP) and further clinical symptoms.
Activation of immune system manifesting as hiperinflammation has been named as cytokine storm19. Many of cytokines are involved in cytokine storm but the one, interleukin-6, is considered as centrally located, in the „eye of the cytokine storm”20.
Among 7 tested APPs, only HAP and AGP, which are moderately reactive APPs did not change in the condition of cytokine storm. PCT showed the greatest increase, an over fourfold, followed by CRP, an almost fourfold increase. We have already pointed out a possible cause for the persistent increase in PCT concentration in the second sample16. This is how the greatest increase in PCT concentration can be explained by the sum of glycoprotein released from other internal organs affected by the disease. The assessment of correlation confirmed the relationship between the intensity of the cytokine storm and the concentration of APPs. Although the correlation of IL-6 with HAP and AGP was also obtained, the correlation coefficient values were low and therefore, in a bilateral comparison, no change in the concentration of these two glycoproteins was obtained in patients with cytokine storm.
The analysis of glycoproteins concentrations depending on the type of oxygen therapy showed that the decisions made (sample B1) were correct, as patients with higher glycoproteins concentrations and, consequently, greater intensity of the cytokine storm were treated with increasingly intense oxygen therapy. The rightness of the decision on the intensity of oxygen therapy was confirmed in a study carried out in second samples, in which, apart from CRP, no differences in glycoprotein concentrations were found.
The oxygen therapy did not affect the concentrations of APPs with the exception of CRP, although it causes an increase in hemoglobin saturation. The increase in hemoglobin saturation by the supply of oxygen does not affect the synthesis of TRF and FER during the time period between samples. Hemoglobin blood levels generally increase after 2 to 4 weeks of iron supplementation and, consequently, the iron stores measured by FER level reaches a normal level around three months. An increase in the concentration of FER (a positive APP) and a decrease in the concentration of TRF (a negative APP) results exclusively from impaired liver function in the acute phase reaction. In such a short time, no changes in the concentration of APPs involved in iron metabolism should be expected.
Among the tested glycoproteins, only two, PCT and TRF, showed the ability to predict the survival of patients suffering from COVID-19. In this respect, our results differ from those obtained by others, who additionally pointed to FER and CRP12,13. But these authors concluded about the prognostic value of APPs based on the relationship between the concentration of acute APPs and the severity of the disease, and not, as we did, on the basis of the difference in the concentration between surviving and non-surviving patients. It should be noted that these studies also showed a relationship between the concentration of APPs such as CRP, PCT and FER and the severity of the disease. Other authors, like us, based their conclusions on difference in FER levels between the groups of survivors and non-survivors10,21.
The limitation of the study include the low number of subjects, although on the other hand, not all COVID-19 studies are carried out on the groups of thousands of patients. Some of them are cited in this work1,11,12,13. In addition, the time interval between the first and second samples were not constant. This resulted from the individual course of the disease and medical decision made. Finally, the numbers of patients divided according to disease severity are not equal. For this reason, the number of patients in severe and critical condition differed significantly from the number of patients in mild condition. This, in turn, resulted from the fact that we qualified for the study subsequent patients admitted to the ward and not selected ones.
Conclusion
In conclusion, the concentration of not all APPs depends on the severity of COVID-19 disease, presence of cytokine storm, the used of oxygen therapy and only some of them are related with the survival outcomes. Among the newly tested APPs only transferrin shows significance as a marker of disease severity and mortality in patients with COVID-19 disease.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Li, L. & Chen, C. Contribution of acute-phase reaction proteins to the diagnosis and treatment of 2019 novel coronavirus disease (COVID-19). Epidemiol. Infect. 148, e164 (2020).
Ehlting, C., Wolf, S. D. & Bode, J. G. Acute-phase protein synthesis: A key feature of innate immune functions of the liver. Biol. Chem. 402, 1129–1145 (2021).
Dowton, S. B. & Colten, H. R. Acute phase reactants in inflammation and infection. Semin. Hematol. 25, 84–90 (1988).
Kushner, I., Gewurz, H. & Benson, M. D. C-reactive protein and the acute-phase response. J. Lab. Clin. Med. 97, 739–749 (1981).
Ingenbleek, Y. & Young, V. Transthyretin (prealbumin) in health and disease: Nutritional implications. Annu. Rev. Nutr. 14, 495–533 (1994).
Fazal, M. C-reactive protein a promising biomarker of COVID-19 severity. Korean J. Clin. Lab. Sci. 53, 201–207 (2021).
Luan, Y. Y., Yin, C. H. & Yao, Y. M. Update advances on C-reactive protein in COVID-19 and other viral infections. Front. Immunol. 12, 720363 (2021).
Kappert, K., Jahić, A. & Tauber, R. Assessment of serum ferritin as a biomarker in COVID-19: bystander or participant? Insights by comparison with other infectious and non-infectious diseases. Biomarkers 25, 616–625 (2020).
Huang, I., Pranata, R., Lim, M. A., Oehadian, A. & Alisjahbana, B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: A meta-analysis. Ther. Adv. Respir. Dis. 14, 1753466620937175 (2020).
Liu, F. et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 127, 104370 (2020).
Topcu, H. & Arik, Y. E. The importance of D-dimer, ferritin, CRP and lymphocyte values in determining mortality in COVID-19 disease in Turkey. Clin. Lab. https://doi.org/10.7754/Clin.Lab.2021.210720 (2022).
Yormaz, B. et al. The evaluation of prognostic value of acute phase reactants in the COVID-19. Bratisl. Lek. Listy 121, 628–633 (2020).
Sayit, A. T., Elmali, M., Deveci, A. & Gedikli, O. Relationship between acute phase reactants and prognosis in patients with or without COVID-19 pneumonia. Rev. Inst. Med. Trop. Sao Paulo 63, e51 (2021).
Giri, N. C. Role of Transferrin in Iron Metabolism [Internet]. Iron Metabolism—A Double-Edged Sword (IntechOpen, 2022). https://doi.org/10.5772/intechopen.100488.
Kim, J. H. Clinical utility of procalcitonin on antibiotic stewardship: A narrative review. Infect. Chemother. 54, 610–620 (2022).
Baj, J. et al. COVID-19: Specific and non-specific clinical manifestations and symptoms: The current state of knowledge. J. Clin. Med. 9, 1753 (2020).
Stenhouse, C., Coates, S., Tivey, M., Allsop, P. & Parker, T. Prospective evaluation of a modified early warning score to aid earlier detection of patients developing critical illness on a general surgical ward. Br. J. Anaesth. 84, 663P (1999).
Subbe, C. P., Kruger, M., Rutherford, P. & Gemmel, L. Validation of a modified early warning score in medical admissions. QJM 94, 521–526 (2001).
Ferrara, J. L., Abhyankar, S. & Gilliland, D. G. Cytokine storm of graft-versus-host disease: A critical effector role for interleukin-1. Transplant. Proc. 25(1 Pt 2), 1216–1217 (1993).
Gubernatorova, E. O., Gorshkova, E. A., Polinova, A. I. & Drutskaya, M. S. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 53, 13–24 (2020).
Orhan, S. et al. The effect of acute phase reactants on the survival of COVID-19 patients in intensive care. Progr. Nutr. 23(S2), e2021268 (2021).
Author information
Authors and Affiliations
Contributions
Conceptualization, L.C., A.P. and B.C.; Methodology, E.G., A.P. and B.C.; Validation, E.G., A.P. and B.C.; Investigation, E.G., B.C., K.G., K.M. and K.M.; Data curation, M.K., M.K. and K.G.; Writing—original draft preparation, E.G., M.K., M.K. and K.G.; Writing-review and editing, L.C., A.P. and B.C.; Supervision, L.C. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chrostek, L., Gan, K., Kazberuk, M. et al. Acute-phase proteins as indicators of disease severity and mortality in COVID-19 patients. Sci Rep 14, 20360 (2024). https://doi.org/10.1038/s41598-024-71325-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71325-2