Abstract
Introduction
We aimed to describe the risk profile of RSV infections among children aged ≤ 24 months in Valladolid from January 2010 to August 2022 and to compare them with influenza and COVID-19 controls.
Methods
We conducted a retrospective cohort study of all laboratory-confirmed RSV, influenza, and COVID-19 infections. We analyzed risk factors for RSV hospitalization and severity (length-of-stay ≥ 8 days, intensive-care-unit admission, in-hospital death or readmission < 30 days) and compared severity between hospitalized RSV patients vs. influenza and COVID-19 controls using multivariable logistic regression models.
Results
We included 1507 patients with RSV (1274 inpatient), 32 with influenza, and 52 COVID-19 controls. Hospitalized RSV (mean age 5.3 months) and COVID-19 (4 months) were younger than influenza (9.1 months) patients. Sixteen percent of patients had RSV within the first month of life. Most infants did not have comorbidities (74% RSV, 56% influenza, and 69% COVID-19). Forty-one percent of patients with RSV and influenza were coinfected vs. 27% COVID-19 (p = 0.04). Among RSV, hospitalization risk factors were prematurity (adjusted OR 3.11 [95% CI 1.66, 4.44]) and coinfection (2.03 [1.45, 2.85]). Risks for higher severity were maternal smoking (1.89 [1.07, 3.33]), prematurity (2.31 [1.59, 3.34]), chronic lung disease (2.20 [1.06, 4.58]), neurodevelopmental condition (4.28 [2.10, 8.73]), and coinfection (2.67 [2.09, 3.40]). Breastfeeding was protective against hospitalization (0.87 [0.80, 0.95]) and severity (0.81 [0.74, 0.88]), while complete vaccination schedule was protective against severity (0.51 [0.27, 0.97]). RSV had 2.47 (1.03, 5.96) higher risk of experiencing any severe outcome compared to influenza and did not show significant differences vs. COVID-19.
Conclusions
RSV hospitalizations were more frequent and severe than influenza, while severity was comparable to the early pandemic COVID-19. Currently, both influenza and COVID-19 vaccines are included in the maternal and childhood Spanish immunization schedule between the ages of 6 and 59 months. RSV monoclonal antibody is recommended for ≤ 6 months but a third of patients were aged 6–24 months. Maternal RSV vaccination can protect their children directly from birth and indirectly through breastfeeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We described the clinical profile of 1507 PCR-confirmed RSV cases (1274 hospitalized) among children aged ≤ 24 months, and compared them with 32 influenza and 52 COVID-19 hospitalized controls. |
Patients with RSV and COVID-19 were younger than influenza patients; 16% of RSV infections happened within the first month of life; 71%, 77% and 38% of infants, respectively, were aged ≤ 6 months. |
Among patients with RSV, those born prematurely or coinfected were more likely to be hospitalized, while breastfeeding was protective against hospitalization. Also, those exposed to maternal smoking, born prematurely, coinfected, or having a chronic lung or neurodevelopmental condition were more likely to experience severe outcomes, while breastfeeding and complete vaccination schedule were protective. |
RSV infection led to more severe outcomes than influenza but did not show statistically significant differences in severity compared to early pandemic COVID-19. |
Currently, both influenza and COVID-19 vaccines are included in the maternal and childhood Spanish immunization schedule between 6 and 59 months of age. RSV monoclonal antibody is recommended for ≤ 6 months but a third of patients were aged 6–24 months. RSV maternal vaccination can protect their children directly from birth and indirectly through breastfeeding. |
Introduction
Respiratory syncytial virus (RSV) is the most common cause of acute lower respiratory infection and contributes substantially to the morbidity and mortality burden globally in children aged ≤ 5 years, especially during the first 6 months of life [1, 2]. In Spain, the 2019 hospitalization rate per 100,000 was 4150 in 0–5 months old, 1251 in 6–11 months old, 486 in 12–23 months old, and 80 in 2–5 years old age groups; whereas the RSV-attributable rates were between 1.3 and 6.5 times higher than those based on standard-of-care RSV-specific codes [3]. Otherwise healthy children account for over 90% of hospitalizations but preterm infants concentrate the higher proportional burden [4, 5]. It has been described that the worst clinical disease progression in infants aged < 6 months is related to lower exposure of the pregnant woman to the RSV epidemic [6].
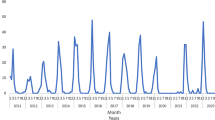
Despite the significant drop in RSV incidence during the autumn–winter of 2020 due to the Coronavirus disease 2019 (COVID-19) pandemic, RSV began to circulate again months earlier than usual in spring–summer of 2021, and cause more clinical disease among older children [7,8,9,10,11]. Nevertheless, in the following 2021–2022 and 2022–2023 seasons, a re-establishment of the pre-pandemic circulation pattern was seen [12].
Traditional RSV prevention strategies focus on non-pharmaceutical interventions such as hand and respiratory hygiene, avoiding contact with infected people, and promoting breastfeeding [13, 14]. Recently, RSV prevention measures have entered a new era with the approval of active and passive immunization strategies following the discovery of the F-protein prefusion configuration [15]. Several RSV vaccines targeting this antigen are being developed for children and adults, including pregnant women [16,17,18]. While no approved vaccines for RSV in infants are currently available, the prevention of infections through licensed maternal vaccination is a cost-effective option [19, 20]. Moreover, in November 2022, the European Medical Agency licensed the monoclonal antibody (mAb) nirsevimab [21], which was approved in Spain on May 2023 and is currently recommended for all infants aged ≤ 6 months and high-risk children from 6–24 months [22].
Since several new RSV preventive strategies are available and public health policies being revised on an ongoing basis, we aimed to provide information to decision-makers and describe the demographic, clinical, and microbiological characteristics of all laboratory-confirmed RSV infections among children aged ≤ 24 months in Valladolid during a 12-year period, and to compare them with influenza and COVID-19 controls. Two infections for which preventive vaccines are already included in the Spanish maternal and childhood immunization schedule.
Methods
Study Design
This was a retrospective cohort study of all laboratory-confirmed RSV infections among children aged ≤ 24 months in Valladolid between January 1, 2010 and August 31, 2022. We identified influenza and COVID-19 hospitalized controls in the same database. We first described the demographic, clinical, and microbiological characteristics of all patients with RSV and influenza and COVID-19 controls, and then performed several comparisons: (1) Between hospitalized and non-hospitalized patients with RSV, we identified risk factors for hospitalization; (2) Among hospitalized patients with RSV, we identified risks for higher severity; (3) Between patients with RSV and influenza, we compared severe clinical outcomes; and (4) Between patients with RSV and COVID-19, we compared severe clinical outcomes.
Study Setting
The study was conducted at two tertiary-care hospitals which cover the entire population of Valladolid, approximately 520,000 inhabitants, including 18,000 (3%) children between 0 and 24 months of age.
Data Sources and Data Management
All patients with polymerase chain reaction (PCR) viral diagnoses were identified from the MICROB database, which is the centralized database for microbiological testing of the public hospitals in the city of Valladolid, Spain. It contains all in-patient and out-patient diagnostic results. The multiplex-type molecular methods used during the study period were: Luminex NxTAG Respiratory Pathogens™ (Luminex, USA), Biomerieux FilmArray RP™ (Biomerieux, France), GenXpert Influenza, RSV rapid diagnostic™ (Cepheid, USA) and Seegene AllPlex Respiratory Panel™ (Seegene, South Korea). These panels identify the following respiratory pathogens: RSV A and B; Influenza A, B, H1 and H3; Human CoV-OC43, NL63, HKU1 and 229E; Parainfluenza 1, 2, 3 and 4; Human Metapneumovirus; Human Bocavirus; Adenovirus; Rhinovirus/Enterovirus; Chlamydophila pneumoniae; Mycoplasma pneumonia; Legionella pneumophila, Bordetella pertussis, and parapertussis. Microbiological records were linked with individual primary care and/or hospital electronic medical records. Data were anonymized and then transferred into a standardized data collection instrument.
Study Population
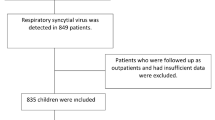
The MICROB database was used to systematically identify all patients aged ≤ 24 months with a positive result for RSV, influenza, or SARS-CoV-2 diagnosed as part of standard-of-care between January 1, 2010 and August 31, 2022. After identifying in-patients and out-patients with RSV, all influenza and COVID-19 hospitalized controls available in the dataset were included. Index date was viral diagnosis date. Influenza and COVID-19 controls were those in the same age range and hospitalized during the same period. If a patient had both infections in the same hospitalization episode (RSV and influenza or RSV and COVID-19), it was excluded from the respective comparative analysis. If a patient with RSV was hospitalized more than once during the study period, only the first hospitalization was considered.
Study Variables
Laboratory and medical records were reviewed and manually abstracted by trained staff. Data were collected in terms of patient demographics (age in months at diagnosis and sex), vaccination status (complete or incomplete for patient’s age based on national immunization calendar [23]), influenza vaccination status, breastfeeding present at time of diagnosis, exclusive breastfeeding time (each month of exclusive breastfeeding counted as 1, each month of mixed breastfeeding and formula counted as 0.5), maternal smoker status, prematurity (< 37 weeks of gestational age), low weight at birth (< percentile 3), low weight at admission (< percentile 3), presence and type of immunocompromising condition, chronic lung disease, cardiovascular or cerebrovascular condition, kidney disease, liver disease, endocrine or metabolic disorder, neurodevelopmental condition, or coinfection; presenting signs and symptoms; laboratory, images, and microbiology results (including anti-infective treatments); and outcomes including hospital length of stay, admission to intensive care unit (ICU) and length of stay, mechanical ventilation and length of use, in-hospital death and readmission to hospital. Variables are detailed in Tables 1 and 2.
For the comparative study, independent variables collected were: age, sex, vaccination completion status, influenza vaccination status, maternal smoker status, exclusive breastfeeding time, prematurity, low weight at birth, low weight at admission, at least one comorbidity (by type), or coinfection. All risk factors and comorbidities were categorized as yes if they were mentioned in the medical chart.
Outcomes were either hospitalization or a composite indicator of severity, defined by the presence of any of these parameters: extended length of hospital stay (length ≥ mean plus 2 days [i.e., 8 days]), ICU admission, in-hospital death, or readmission to hospital within 30 days.
Statistical Methods
For the descriptive part of the study, demographics, clinical and microbiological characteristics, and outcomes were described separately for each viral etiology (RSV, influenza, SARS-CoV-2). Significance was tested using two-sided chi-square or Fisher’s exact tests (categorical variables) and Student’s t test or Mann–Whitney U tests (continuous variables), p value < 0.05 was considered statistically significant.
For the comparative part of the study, a series of multivariable regression models were used to identify risk factors for hospitalization and for experiencing any severe outcome among patients with RSV, and to compare severity between patients with RSV vs. influenza and COVID-19. Odds ratios (ORs) and their 95% confidence intervals (CIs) were calculated. A stepwise approach with both entry and exit level p values < 0.1 was used in a multivariable logistic regression model to identify potential confounders and to determine risk factors associated with disease outcomes. After the statistically significant covariates were selected by this stepwise approach, they were combined with the clinically significant covariates (such as age) into the final analyses. Only patients with complete data for models were analyzed, missing data were not imputed. All data were processed and analyzed using SAS Studio, an online interface for SAS Version 9.04.01M7P08062020 (release date March 16, 2023).
Ethical Considerations
This study was approved by the Ethics Committee of the Hospital Clínico Universitario de Valladolid under the code PI-22-2729 and the Hospital Río Hortega under the code 22-PI096, both approvals included a waiver for the need of informed consent.
Similar methodology has been presented previously [24], as this is a similar analysis but with a different age population.
Results
We analyzed 1507 patients with RSV, including 1274 hospitalized and 233 outpatients. For the hospitalized patients, we identified 32 influenza and 52 COVID-19 controls (of which five were from 2020 [Wuhan and Alfa were the main circulating variants], 18 from 2021 [Alfa and Delta] and 29 from 2022 [Omicron]). Among all RSV infections, 168 (13%) had a type identified: 55% A, 44% B, and 1% A + B. While during 2010–2018 subtype testing was rare, subtype A was more frequent in 2019 (82.6%) and 2020 (76.9%), and subtype B prevailed in 2021 (79.1%) and 2022 (70.0%) (Supplementary Table 1). Patients with influenza had this infection coded as primary diagnosis except for two patients (choking episode and urinary tract infection), while those with COVID-19 had this infection coded as primary except for seven patients (idiopathic thrombocytopenic purpura, dermoid cyst surgical intervention, subdural empyema, Williams–Beuren syndrome, cytomegalovirus infection, and two with urinary tract infection).
Comparison of Clinical Profile Between RSV and Influenza and COVID-19
Hospitalized patients with RSV were of similar age as those with COVID-19 but significantly younger than patients with influenza (mean age 5.3, 4.0, and 9.1 months, respectively). Patients aged ≤ 6 months represented 71, 77, and 38%, respectively (Fig. 1); while among RSV-positive, those aged ≤ 12 weeks represented 47% (16% were ≤ 4 weeks) (Fig. 2). Patients with RSV were more frequently breastfed at the time of infection diagnosis and were more likely to have a completed vaccination schedule for their age compared to patients with COVID-19 (45.5% vs. 26.9%, p = 0.0099 and 92.9% vs. 73.1%, p = < 0.0001; respectively) (Table 1).
Most children did not have comorbidities: 74.3% RSV, 56.2% influenza, and 69.2% COVID-19. The most frequent comorbidities among patients with RSV were cardiovascular (4.5%), followed by chronic lung (3.4%), and immunocompromising (2.0%) conditions. Patients with RSV were less frequently premature compared to those with influenza (13.1% vs. 34.4%, p = 0.0021), had less kidney disease compared to those with COVID-19 (1.2% vs. 5.8%, p = 0.0354), and had less chronic lung diseases compared to both (RSV 3.4%, influenza 12.5%, and COVID-19 11.5%, p = 0.0253 vs. influenza and p = 0.0104 vs. COVID-19) (Table 2).
More than 40% of patients with RSV and influenza had a coinfection, significantly more than COVID-19 (26.9%, p = 0.0443). From those with pathogen diagnosis date available (N = 549), 18.8% (RSV) and 30.0% (influenza) were nosocomial infections, i.e., diagnosed more than 48 h after hospitalization. RSV coinfection was more frequent with another virus (16.6%), bacteria (14.8%), and bacteria and virus (8.3%) (Table 1). The most frequent viruses were rhinovirus/enterovirus (13.0%), bocavirus (4.9%), coronavirus (4.2%), adenovirus (4.0%), and parainfluenza (3.3%); while the most frequent bacteria were Haemophilus influenzae (10.8%), Staphylococcus aureus (5.0%), Escherichia coli (3.6%), Moraxella catarrhalis (2.7%), and Streptococcus pneumoniae (1.7%) (data not shown).
At hospital admission, patients with RSV were those who significantly showed most signs of acute lower respiratory tract infection (88.7% vs. 56.3% influenza and 36.5% COVID-19, p = < 0.0001)—marked by dyspnea and wheezing—and upper respiratory tract infection (86.6% vs. 71.9% influenza and 51.9%, COVID-19, p = 0.0315 and p < 0.0001, respectively)—predominantly cough and rhinorrhea. They also had the most gastrointestinal signs (64.2% RSV vs. 37.5% influenza and 44.2% COVID-19, p = 0.0027 and p = 0.0048, respectively)—mainly decreased appetite. Conversely, neurological symptoms were highest in patients with influenza (0.9% RSV vs. 9.4%, p = 0.0050) (Table 2).
Images showed higher pulmonary infiltrates in patients with RSV compared to COVID-19 (11.4% vs. 0%, p = 0.0049) (Table 2). Among RSV, 53.7% of infiltrates were bilateral, 82.4% had an interstitial (vs. alveolar) and 52.7% a focal (vs. diffuse) pattern (Supplementary Table 2).
Laboratory parameters of inflammation were more frequent in RSV compared to COVID-19-infected children, i.e., thrombocytosis (37.4% vs. 21.2%, p = < 0.0184), leukocytosis (31.7% vs. 12%, p = 0.0018), neutrophilia (16.0% vs. 1.9%, p = 0.0027) and elevated C-reactive protein (44.4% vs. 7.7%, p = < 0.0001). Hypoxemia was also significantly more frequent among RSV (16.8% vs. 3.9% COVID-19, p = 0.0112) (Table 2). A third of patients with RSV (n = 402) received antibiotics, although 51.7% (n = 208) of them did not have a bacterial coinfection (Supplementary Table 3).
RSV Risk Factors for Hospitalization
Significant risk factors for RSV hospitalization in the multivariate analysis were prematurity (adjusted OR 3.11 [95% CI 1.67, 5.80]) and coinfection (2.03 [1.45, 2.86]). While exclusive breastfeeding time was protective: for each additional month of breastfeeding, there was a reduction of 13% (5–20%) in the risk of hospitalization (Table 3).
RSV Risk Factors for Severity
The fatality rate among hospitalized patients with RSV was 0.24%. For these patients, significant risk factors associated with severity in the multivariate analysis were maternal smoking (adjusted OR 1.89 [95% CI 1.08, 3.33]), prematurity (2.31 [1.60, 3.34]), chronic lung disease (2.21 [1.06, 4.58]), neurodevelopmental condition (4.29 [2.11, 8.73]) and coinfection (2.67 [2.10, 3.40]). While vaccination complete for patient’s age (0.51 [0.27, 0.97]) and breastfeeding time were protective: for each additional month of exclusive breastfeeding there was a reduction of 19% (12–26%) in the risk of suffering any severe outcome (Table 4). RSV type was not associated with higher severity (A vs. B, adjusted OR 1.08 [0.53, 2.19]) (Supplementary Table 4).
Comparison of Severity Between RSV and Influenza and COVID-19
Whereas patients with RSV and COVID-19 did not show differences in any severity parameters, RSV cases were more than twice as likely (adjusted OR 2.48 [1.03, 5.96]) to suffer any severe outcome compared to influenza cases (Table 5).
Discussion
This comprehensive analysis of individual medical health records in a large sample of the general infant and toddler population provides valuable insights into the demographic and clinical profile, risk factors, and outcomes of RSV infections. In addition, we have compared hospitalized patients with RSV to influenza and COVID-19 controls. Two infections for which preventive vaccines are already included in the Spanish maternal and childhood immunization schedule: influenza vaccination is recommended for pregnant women and all children from 6–59 months of age, and COVID-19 vaccine for pregnant women and high-risk children [23, 25].
As expected, there were significantly fewer hospitalized patients with influenza and COVID-19 compared to RSV [26,27,28]. Also, patients with RSV and COVID-19 were younger than those with influenza [27,28,29]. Even though the higher incidence and severity occur in infants aged < 6 months, and this is why mAbs have been first restricted to this group, in our analysis patients with RSV aged 6–24 months represented a third of overall RSV population, highlighting the need for extending mAbs or future vaccines recommendation to this group. In addition, 16% of patients with RSV were aged ≤ 4 weeks. Depending on when mAbs are administered after birth and with the uncertainty of how long after administration are actually effective–since efficacy data is from administration date and not based on age—[21] there might be a time window within this first month of life when infants remain vulnerable to RSV infection. This is not a concern for maternal RSV vaccination, which demonstrated to protect from birth in the phase 3 study (i.e., the first severe case occurred in the placebo group in an 8-day-old infant) [16].
About 26% of children with RSV, 31% with COVID-19, and 44% with influenza had comorbidities (6% of those with influenza and 13% of those with COVID-19 had other cause coded as primary diagnosis). This comorbidity profile is slightly higher than reported in previous RSV studies [4, 30, 31], but was as expected for patients with influenza and COVID-19 [8, 27, 28]. A coinfection was present in 40% of patients with RSV and influenza, similar to what has been described [27, 32,33,34], and this proportion was significantly higher than in patients with COVID-19. A large proportion of these coinfections was nosocomial infections, especially among patients with influenza, highlighting the need for effective infection-control procedures and policies. Moreover, a third of patients with RSV received antibiotic treatment, although the majority did not have a bacterial coinfection, reinforcing the message that more effort should be made to reduce antibiotic prescriptions in children with viral infections, besides the need for molecular testing to identify the etiology of the infection [27, 35].
Patients with RSV showed higher acute respiratory tract infection signs and symptoms than those with influenza or COVID-19. This is in line with previous studies reporting that while RSV infection is more likely to present as a clinical respiratory syndrome with cough, rhinitis, dyspnea, and wheezing. COVID-19 infection presents as a general disease dominated by fever with or without gastrointestinal and neurological signs and symptoms [8, 26, 29, 36].
Laboratory parameters of inflammation and hypoxemia were similar between RSV and influenza but less frequently elevated in patients with COVID-19. Whereas it has been described that RSV and influenza infections present with lymphocytosis, lymphopenia caused by the inhibitory effect of activated cytokines seems to be consistently more frequent in COVID-19 infections [8, 29, 36, 37]. Although we have found less infiltrates in the images of patients with SARS-CoV-2 [38], other studies did not find such differences [8, 37].
Regarding factors associated with higher severity in the clinical progression of RSV, we have found that maternal smoking, prematurity, chronic lung disease, neurodevelopmental condition, and coinfections increased the risk, which is corroborated by many studies [2, 30, 35, 39,40,41,42,43,44,45], although we did not find an association with male sex and low weight at birth, as previously described [30, 31, 41, 42]. Concerning coinfections, a recent systematic review found no association between viral coinfections and severity, except for RSV and human metapneumovirus coinfections, which were associated with an increased risk of ICU admission [46]. This difference could be due to the fact that we have included bacterial and fungal coinfections as well.
In our study, RSV cases were more than twice as likely to suffer any severe outcome compared to influenza cases and this is in line with what has been described [27, 47]. Nevertheless, whereas in our analysis there were no statistically significant differences between RSV vs. COVID-19 in severity and this is confirmed by some studies [8, 29], others have reported higher severity of RSV [26, 27, 36,37,38, 48]. These differences could be likely due to methodological issues, with the addition that main SARS-CoV-2 circulating variant must also be considered: our controls were from the early phase of the pandemic, when more virulent variants were circulating. Reproducing such a study at present would likely show lower disease severity than in our analysis since it decreased significantly as the pandemic progressed [49,50,51,52,53].
RSV subtype was not associated with severity in our analysis, although results must be taken with caution since only 13% of patients had a subtype identified and it contradicts previous studies reporting higher severity of subtype A [54,55,56,57]. In addition, both subtypes were co-circulating in each year, showing a biennial dominance pattern (higher A in 2019–2020 vs. higher B in 2021–2022). Serotype and genotype analyses have relevant implications for understanding vaccines and antivirals effectiveness, so they should be part of the surveillance system [9, 54, 58, 59].
Examining the risk factors for hospitalization amongst patients with RSV, we found that they were more than twice as likely to be hospitalized when they were born premature or had a coinfection [60]. This is in line with a meta-analysis reporting that preterm infants have a disproportionately high burden of RSV disease, accounting for a quarter of the overall hospitalization burden, and that early preterm newborns have a substantial burden persisting into the second year of life [2].
In our study, exclusive breastfeeding time was protective against hospitalization and higher severity. This is corroborated by a meta-analysis evaluating 19 peer-reviewed studies that included 16,787 infants from 31 countries, which found that non-breastfeeding practices pose a significant risk for severe RSV-associated acute lower respiratory tract infection and hospitalization. Exclusive breastfeeding for more than 4 to 6 months significantly lowered admission rates, length of hospital stay, supplemental oxygen use, and admission to ICU [61], while another meta-analysis showed a reduced risk of RSV infection of 27% (10–42%) if the infant was breastfed [40]. In Spain, a study reported that not having been breastfed for at least 1 month was predictive for ICU admission and not having been breastfed for at least 2 months was predictive of needing mechanical ventilation [45]. Another study from Nepal concluded that prefusion RSV fusion protein IgG antibodies level in breast milk seems to be correlated with protection against the disease, and that one mechanism of action of the maternal RSV vaccination could be through the induction of these antibodies [17].
Our study had several limitations. First, the potential of missing or inaccurate data due to the retrospective nature of the study. Second, the lack of other socioeconomic or environmental confounders included for the same data source limitation. Last, the low number of influenza and COVID-19 controls: even though we have included all available cases, the hospitalization rate in this age group is low and primary cause of hospitalization could be other than the infection per se. This small sample size reduces the statistical power of the study and limits the possibility of obtaining robust conclusions. In contrast, the main strengths of our study are the large number of RSV cases and extensive study period (11 seasons) covered, the granularity of the clinical and microbiological data analyzed, and that it is one of the very few studies comparing RSV with influenza or SARS-CoV-2 infections in infants and toddlers.
In summary, among children aged ≤ 24 months, RSV hospitalizations were more frequent and severe than influenza, while severity was comparable to the early pandemic of COVID-19. RSV mAb is currently recommended for infants ≤ 6 months of age. Even though both influenza and COVID-19 vaccines are currently included in the national immunization schedule for those aged 6–24 months, RSV immunization is still to be implemented, considering that a third of RSV patients were in this age group. RSV maternal vaccination can protect their children directly from birth and indirectly through breastfeeding.
Data availability
The datasets analyzed during the current study are not publicly available.
References
Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–64.
Wang X, Li Y, Shi T, Bont LJ, Chu HY, Zar HJ, et al. Global disease burden of and risk factors for acute lower respiratory infections caused by respiratory syncytial virus in preterm infants and young children in 2019: a systematic review and meta-analysis of aggregated and individual participant data. Lancet Lond Engl. 2024;403(10433):1241–53.
Haeberer M, Bruyndonckx R, Polkowska-Kramek A, Torres A, Liang C, Nuttens C, et al. Estimated respiratory syncytial virus-related hospitalizations and deaths among children and adults in Spain, 2016–2019. Infect Dis Ther. 2024;13(3):463–80.
Martinón-Torres F, Carmo M, Platero L, Drago G, López-Belmonte JL, Bangert M, et al. Clinical and economic hospital burden of acute respiratory infection (BARI) due to respiratory syncytial virus in Spanish children, 2015–2018. BMC Infect Dis. 2023;23(1):385.
Sanchez-Luna M, Elola FJ, Fernandez-Perez C, Bernal JL, Lopez-Pineda A. Trends in respiratory syncytial virus bronchiolitis hospitalizations in children less than 1 year: 2004–2012. Curr Med Res Opin. 2016;32(4):693–8.
Ramos-Fernández JM, Hernández-Yuste A, Gutiérrez-Bedmar M, Cordón Martínez AM, Moreno-Pérez D. Does exposure of pregnant women to epidemic respiratory syncytial virus affect the severity of bronchiolitis? Enfermedades Infecc Microbiol Clínica. 2019;37(4):251–5.
Suss RJ, Simões EAF. Respiratory syncytial virus hospital-based burden of disease in children younger than 5 years, 2015–2022. JAMA Netw Open. 2024;7(4): e247125.
Nunziata F, Salomone S, Catzola A, Poeta M, Pagano F, Punzi L, et al. Clinical presentation and severity of SARS-CoV-2 infection compared to respiratory syncytial virus and other viral respiratory infections in children less than two years of age. Viruses. 2023;15(3):717.
Piñana M, González-Sánchez A, Andrés C, Vila J, Creus-Costa A, Prats-Méndez I, et al. Genomic evolution of human respiratory syncytial virus during a decade (2013–2023): bridging the path to monoclonal antibody surveillance. J Infect. 2024;88(5): 106153.
Guerrero-del-Cueto F, Ramos-Fernandez JM, Leiva-Gea I, Reina-Moreno E, Ortiz-Ortigosa A, Carazo-Gallego B, et al. Bronchiolitis before and after the SARS-CoV-2 pandemic: twelve years of experience in a Spanish paediatric hospital. Pediatr Pulmonol. 2023;58(4):1201–9.
Rodríguez-Fernández R, González-Martínez F, Perez-Moreno J, González-Sánchez MI, De La Mata NS, Toledo Del Castillo B, et al. Clinical phenotype of respiratory syncytial virus bronchiolitis before and during the coronavirus disease 2019 pandemic. Am J Perinatol. 2022;2:2.
Olguin TCP, Mazagatos C, Galindo-Carretero S, Vega-Piris L, Lozano-Álvarez M, Pérez-Gimeno G, et al. Epidemiología y carga de enfermedad por VRS en España. SiVIRA, temporadas 2021–22 y 2022–23. Bol Epidemiológico Sem. 2024;32(1):21–35.
Mineva GM, Purtill H, Dunne CP, Philip RK. Impact of breastfeeding on the incidence and severity of respiratory syncytial virus (RSV)-associated acute lower respiratory infections in infants: a systematic review highlighting the global relevance of primary prevention. BMJ Glob Health. 2023;8(2): e009693.
Dallagiacoma G, Arthur Rhedin S, Odone A, Alfvén T. A comparative analysis of non-pharmaceutical interventions for preventing the respiratory syncytial virus in 30 European countries. Acta Paediatr. 2024;113(6):1388–95.
McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340(6136):1113–7.
Kampmann B, Madhi SA, Munjal I, Simões EAF, Pahud BA, Llapur C, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med. 2023;388(16):1451–64.
Mazur NI, Terstappen J, Baral R, Bardají A, Beutels P, Buchholz UJ, et al. Respiratory syncytial virus prevention within reach: the vaccine and monoclonal antibody landscape. Lancet Infect Dis. 2023;23(1):e2-21.
Mejias A, Rodríguez-Fernández R, Oliva S, Peeples ME, Ramilo O. The journey to a respiratory syncytial virus vaccine. Ann Allergy Asthma Immunol. 2020;125(1):36–46.
Pang Y, Lu H, Cao D, Zhu X, Long Q, Tian F, et al. Efficacy, immunogenicity and safety of respiratory syncytial virus prefusion F vaccine: systematic review and meta-analysis. BMC Public Health. 2024;24(1):1244.
Álvarez Aldean J, Rivero Calle I, Rodríguez Fernández R, Aceituno Mata S, Bellmunt A, Prades M, et al. Cost-effectiveness analysis of maternal immunization with RSVpreF vaccine for the prevention of respiratory syncytial virus among infants in Spain. Infect Dis Ther. 2024;13(6):1315–31.
Hammitt LL, Dagan R, Yuan Y, Baca Cots M, Bosheva M, Madhi SA, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386(9):837–46.
Ministry of Health of Spain. Recomendaciones de utilización de nirsevimab para la temporada 2024–2025 en España [Internet]. 2024 [cited 2024 Jul 3]. Available from: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/comoTrabajamos/docs/Nirsevimab.pdf
Ministry of Health of Spain. National Immunization Calendar [Internet]. 2024. Available from: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/calendario/docs/CalendarioVacunacion_Todalavida.pdf
Haeberer M, Mengel M, Fan R, Toquero-Asensio M, Martin-Toribio A, Liu Q, et al. RSV Risk profile in hospitalized adults and comparison with influenza and COVID-19 controls in Valladolid, Spain, 2010–2022. Infect Dis Ther. 2024. https://doi.org/10.1007/s40121-024-01021-1.
Ministry of Health of Spain. Recomendaciones de vacunación frente a gripe y COVID-19 en la temporada 2023–2024 en España. Actualización [Internet]. 2024. Available from: https://www.sanidad.gob.es/areas/promocionPrevencion/vacunaciones/gripe_covid19/docs/RecomendacionesVacunacion_Gripe-Covid19.pdf
Meyer M, Ruebsteck E, Eifinger F, Klein F, Oberthuer A, Van Koningsbruggen-Rietschel S, et al. Morbidity of respiratory syncytial virus (RSV) infections: RSV compared with severe acute respiratory syndrome coronavirus 2 infections in children aged 0–4 years in Cologne, Germany. J Infect Dis. 2022;226(12):2050–3.
Tripathi S, Al-Sayyed B, Gladfelter TR. Comparative epidemiology, hospital course, and outcomes of viral respiratory infections in hospitalized pediatric patients. Indian J Med Microbiol. 2021;39(1):24–9.
Law A, Sato R, López-Ibáñez de Aldecoa A, Seabroke S, Ramirez Agudelo J, Mora L, Sarabia L, Meroc E, Aponte-Torres Z, Haeberer M. Economic burden of hospitalized respiratory syncytial virus infection among children in Spain, 2016–2019 [Internet]. Available from: https://www.ispor.org/docs/default-source/euro2023/eu-ispor-posterchildren-spain-rsv-cost-studyv4131832-pdf.pdf?sfvrsn=163ce052_0
Miron VD, Raianu RO, Filimon C, Craiu M. Clinical differences between SARS-CoV-2 and RSV infections in infants: findings from a case-control study. Viruses. 2023;16(1):63.
Cilla G, Sarasua A, Montes M, Arostegui N, Vicente D, Pérez-Yarza E, et al. Risk factors for hospitalization due to respiratory syncytial virus infection among infants in the Basque Country. Spain Epidemiol Infect. 2006;134(3):506–13.
Vila J, Lera E, Andrés C, Piñana M, Rello-Saltor V, Tobeña-Rué M, et al. The burden of non-SARS-CoV2 viral lower respiratory tract infections in hospitalized children in Barcelona (Spain): a long-term, clinical, epidemiologic and economic study. Influenza Other Respir Viruses. 2023;17(1): e13085.
Cruz-Cañete M, Moreno-Pérez D, Jurado-Ortiz A, García-Martín FJ, López-Siles J, Olalla-Martín L. El virus de la gripe en pediatría. Un motivo de hospitalización. Enfermedades Infecc Microbiol Clínica. 2007;25(3):177–83.
Diaz-Diaz A, Garcia-Maurino C, Jordan-Villegas A, Naples J, Ramilo O, Mejias A. Viral bacterial interactions in children: impact on clinical outcomes. Pediatr Infect Dis J. 2019;38(6S):S14–9.
Calvo C, Pozo F, García-García M, Sanchez M, Lopez-Valero M, Pérez-Breña P, et al. Detection of new respiratory viruses in hospitalized infants with bronchiolitis: a three-year prospective study. Acta Paediatr. 2010;99(6):883–7.
Piñero Fernández JA, Alfayate Migueléz S, Menasalvas Ruiz A, Salvador García C, Moreno Docón A, Sánchez-Solís De Querol M. Características epidemiológicas, clínicas y terapéuticas de lactantes hospitalizados por bronquiolitis. An Pediatría. 2012;77(6):391–6.
Fedorczak A, Zielińska N, Nosek-Wasilewska P, Mikołajczyk K, Lisiak J, Zeman K, et al. Comparison of COVID-19 and RSV infection courses in infants and children under 36 months hospitalized in paediatric department in fall and winter season 2021/2022. J Clin Med. 2022;11(23):7088.
Jia Z, Yan X, Gao L, Ding S, Bai Y, Zheng Y, et al. Comparison of clinical characteristics among COVID-19 and non-COVID-19 pediatric pneumonias: a multicenter cross-sectional study. Front Cell Infect Microbiol. 2021;1(11): 663884.
Alkan Ozdemir S, Soysal B, Calkavur S, Gökmen Yıldırım T, Kıymet E, Kalkanlı O, et al. Is respiratory syncytial virus infection more dangerous than COVID 19 in the neonatal period? J Matern Fetal Neonatal Med. 2022;35(22):4398–403.
Carbonell-Estrany X, Figueras-Aloy J, Law BJ, Stud IRI por VRSSGICN on I in C. Identifying risk factors for severe respiratory syncytial virus among infants born after 33 through 35 completed weeks of gestation: different methodologies yield consistent findings. Pediatr Infect Dis J. 2004;23(11):193.
Manzoni P, Viora E, Lanari M, Iantomasi R, Montuori EA, Rodgers-Gray B, et al. Maternal risk factors for respiratory syncytial virus lower respiratory tract infection in otherwise healthy preterm and term infants: a systematic review and meta-analysis. Pediatr Infect Dis J [Internet]. 2024 May 15 [cited 2024 Jul 9]; Available from: https://journals.lww.com/https://doi.org/10.1097/INF.0000000000004387
Ramos-Fernández JM, Moreno-Pérez D, Gutiérrez-Bedmar M, Hernández-Yuste A, Cordón-Martínez AM, Milano-Manso G, et al. [Prediction of Severe Course in Infants with RSV Bronchiolitis under 6 Months. Spain]. Rev Esp Salud Publica. 2017;91:e201701006.
Figueras-Aloy J, Manzoni P, Paes B, Simões EAF, Bont L, Checchia PA, et al. Defining the risk and associated morbidity and mortality of severe respiratory syncytial virus infection among preterm infants without chronic lung disease or congenital heart disease. Infect Dis Ther. 2016;5(4):417.
Carbonell-Estrany X, Fullarton JR, Gooch KL, Vo PG, Figueras-Aloy J, Lanari M, et al. Effects of parental and household smoking on the risk of respiratory syncytial virus (RSV) hospitalisation in late-preterm infants and the potential impact of RSV prophylaxis. J Matern Fetal Neonatal Med. 2013;26(9):926–31.
Figueras Aloy J, Quero J. Recomendaciones para la prevención de la infección por virus respiratorio sincitial. An Pediatría. 2005;63(4):357–62.
Calvo C, García-García ML, Blanco C, Vázquez MC, Frías ME, Pérez-Breña P, et al. Multiple simultaneous viral infections in infants with acute respiratory tract infections in Spain. J Clin Virol. 2008;42(3):268–72.
Li Y, Pillai P, Miyake F, Nair H. The role of viral co-infections in the severity of acute respiratory infections among children infected with respiratory syncytial virus (RSV): a systematic review and meta-analysis. J Glob Health. 2020;10(1): 010426.
Rodriguez-Fernandez R, González-Sánchez MI, Perez-Moreno J, González-Martínez F, De La Mata NS, Mejias A, et al. Age and respiratory syncytial virus etiology in bronchiolitis clinical outcomes. J Allergy Clin Immunol Glob. 2022;1(3):91–8.
Brigadoi G, Demarin GC, Boracchini R, Pierantoni L, Rossin S, Barbieri E, et al. Comparison between the viral illness caused by SARS-CoV-2, influenza virus, respiratory syncytial virus and other respiratory viruses in pediatrics. Viruses. 2024;16(2):199.
Peláez A, Ruiz Del Árbol N, Vázquez Sellán A, Castellano JM, Soriano JB, Ancochea J, et al. Clinical characteristics and outcomes among hospitalised COVID-19 patients across epidemic waves in Spain: an unCoVer analysis. Med Clin (Barc). 2024;162(11):523–31.
Bahl A, Mielke N, Johnson S, Desai A, Qu L. Severe COVID-19 outcomes in pediatrics: an observational cohort analysis comparing alpha, delta, and omicron variants. Lancet Reg Health - Am. 2023;18: 100405.
Alteri C, Scutari R, Costabile V, Colagrossi L, La Rosa KY, Agolini E, et al. Publisher Correction: Epidemiological characterization of SARS-CoV-2 variants in children over the four COVID-19 waves and correlation with clinical presentation. Sci Rep. 2022;12(1):12814.
Pino R, Antoñanzas JM, Paredes-Carmona F, Perramon A, Rivière JG, Coma M, et al. Multisystem inflammatory syndrome in children and SARS-CoV-2 variants: a two-year ambispective multicentric cohort study in Catalonia. Spain Eur J Pediatr. 2023;182(4):1897–909.
Wiedenmann M, Ipekci AM, Araujo-Chaveron L, Prajapati N, Lam YT, Alam MI, et al. SARS-CoV-2 variants of concern in children and adolescents with COVID-19: a systematic review. BMJ Open. 2023;13(10): e072280.
Rios-Guzman E, Simons LM, Dean TJ, Agnes F, Pawlowski A, Alisoltanidehkordi A, et al. Deviations in RSV epidemiological patterns and population structures in the United States following the COVID-19 pandemic. Nat Commun. 2024;15(1):3374.
Hall CB, Walsh EE, Schnabel KC, Long CE, McConnochie KM, Hildreth SW, et al. Occurrence of groups A and B of respiratory syncytial virus over 15 years: associated epidemiologic and clinical characteristics in hospitalized and ambulatory children. J Infect Dis. 1990;162(6):1283–90.
Jafri HS, Wu X, Makari D, Henrickson KJ. Distribution of respiratory syncytial virus subtypes A and B among infants presenting to the emergency department with lower respiratory tract infection or apnea. Pediatr Infect Dis J. 2013;32(4):335–40.
Vila J, Lera E, Peremiquel-Trillas P, Andrés C, Martínez L, Barceló I, et al. Increased RSV-A bronchiolitis severity in RSV-infected children admitted to a reference center in Catalonia (Spain) between 2014 and 2018. J Pediatr Infect Dis Soc. 2023;12(3):180–3.
Holmdahl I, Bents SJ, Baker RE, Casalegno JS, Trovão NS, Park SW, et al. Differential impact of COVID-19 non-pharmaceutical interventions on the epidemiological dynamics of respiratory syncytial virus subtypes A and B. Sci Rep. 2024;14(1):14527.
Abu-Raya B, Viñeta Paramo M, Reicherz F, Lavoie PM. Why has the epidemiology of RSV changed during the COVID-19 pandemic? eClinicalMedicine. 2023;61: 102089.
Cebey-López M, Pardo-Seco J, Gómez-Carballa A, Martinón-Torres N, Martinón-Sánchez JM, Justicia-Grande A, et al. Bacteremia in children hospitalized with respiratory syncytial virus infection. PLoS ONE. 2016;11(2): e0146599.
Mineva G, Philip R. Impact of breastfeeding on the incidence and severity of respiratory syncytial virus bronchiolitis in infants: systematic review. Rural Remote Health. 2023;23(1):8088.
Medical writing/editorial assistance
Martin Mengel received funding from Pfizer for the development of the first manuscript draft under the direction of the authors.
Funding
This study was funded by Pfizer Ltd, including the journal's Rapid Service Fee.
Author information
Authors and Affiliations
Contributions
All authors attest to meeting the International Committee of Medical Journal Editors (ICMJE) criteria for authorship. Mariana Haeberer contributed to conceptualization, methodology, data analysis, data review, manuscript writing, and handling all revisions. Martin Mengel developed the first manuscript draft under the direction of the authors. Ivan Sanz-Muñoz contributed to conceptualization and review. Marina Toquero Asensio and Alejandro Martín Toribio were responsible for data abstraction. Rong Fan, Yongzheng He, and Qing Liu performed data analysis. Jessica Atwell, Sonal Uppal, José M. Eiros and Javier Castrodeza Sanz reviewed the manuscript. Silvia Rojo-Rello, Marta Domínguez-Gil, Cristina Hernán-García, and Virginia Fernández-Espinilla provided the study data.
Corresponding author
Ethics declarations
Conflicts of interests
Mariana Haeberer, Rong Fan, Qing Liu, Sonal Uppal and Jessica Atwell are employees of Pfizer and may own Pfizer stock. Marina Toquero Asensio, Alejandro Martín Toribio, Silvia Rojo Rello, Marta Domínguez Gil, Cristina Hernán García, Virginia Fernández Espinilla, José M. Eiros, Javier Castrodeza Sanz, and Ivan Sanz-Muñoz received funding from Pfizer for data abstraction, protocol, and manuscript review. Alejandro Martín Toribio received funding by the call for UVa 2023 predoctoral contracts, co-funded by Banco Santander.
Ethical considerations
This study was approved by the Ethics Committee of the Hospital Clínico Universitario de Valladolid under the code PI-22-2729 and the Hospital Río Hortega under the code 22-PI096, both approvals included a waiver for the need of informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Prior presentation: European Respiratory Society (ERS) Congress, Vienna-Austria, 10th September 2024.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Haeberer, M., Mengel, M., Fan, R. et al. Respiratory Syncytial Virus Risk Profile in Hospitalized Infants and Comparison with Influenza and COVID-19 Controls in Valladolid, Spain, 2010–2022. Infect Dis Ther 13, 2395–2413 (2024). https://doi.org/10.1007/s40121-024-01058-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-024-01058-2