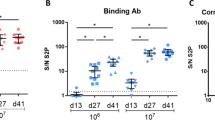
We studied a needle-free jet injection delivery of an experimental mRNA vaccine encoding the receptor-binding domain of the SARS-CoV-2 S protein (mRNA-RBD). Immunization of BALB/c mice with mRNA-RBD by a needle-free jet injector induced high levels of antibodies with virus-neutralizing activity and a virus-specific T-cell response. The immune response was low in the group of mice that received intramuscular injection of mRNA-RBD. The effectiveness of this simple and safe method of mRNA delivering has been demonstrated. Thus, jet injection of mRNA vaccine can be a good alternative to lipid nanoparticles.
Similar content being viewed by others
References
Wilson B, Geetha KM. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J. Drug Deliv. Sci. Technol. 2022;74:103553. https://doi.org/10.1016/j.jddst.2022.103553
Eygeris Y, Gupta M, Kim J, Sahay G. Chemistry of lipid nanoparticles for RNA delivery. Acc. Chem. Res. 2022;55(1):2-12. https://doi.org/10.1021/acs.accounts.1c00544
Huang Q, Zeng J, Yan J. COVID-19 mRNA vaccines. J. Genet. Genomics. 2021;48(2):107-114. https://doi.org/10.1016/j.jgg.2021.02.006
Banerji A, Wickner PG, Saff R, Stone CAJr, Robinson LB, Long AA, Wolfson AR, Williams P, Khan DA, Phillips E, Blumenthal KG. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J. Allergy Clin. Immunol. Pract. 2021;9(4):1423-1437. https://doi.org/10.1016/j.jaip.2020.12.047
Aldosari BN, Alfagih IM, Almurshedi AS. Lipid nanoparticles as delivery systems for RNA-based vaccines. Pharmaceutics. 2021;13(2):206. https://doi.org/10.3390/pharmaceutics13020206
Islam MA, Reesor EK, Xu Y, Zope HR, Zetter BR, Shi J. Biomaterials for mRNA delivery. Biomater. Sci. 2015;3(12):1519-1533. https://doi.org/10.1039/c5bm00198f
Karpenko LI, Rudometov AP, Sharabrin SV, Shcherbakov DN, Borgoyakova MB, Bazhan SI, Volosnikova EA, Rudometova NB, Orlova LA, Pyshnaya IA, Zaitsev BN, Volkova NV, Azaev MS, Zaykovskaya AV, Pyankov OV, Ilyichev AA. Delivery of mRNA vaccine against SARS-CoV-2 using a polyglucin:spermidine conjugate. Vaccines (Basel). 2021;9(2):76. https://doi.org/10.3390/vaccines9020076
Guan S, Rosenecker J. Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Ther. 2017;24(3):133-143. https://doi.org/10.1038/gt.2017.5
Momin T, Kansagra K, Patel H, Sharma S, Sharma B, Patel J, Mittal R, Sanmukhani J, Maithal K, Dey A, Chandra H, Rajanathan CT, Pericherla HP, Kumar P, Narkhede A, Parmar D. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine. 2021;38:101020. https://doi.org/10.1016/j.eclinm.2021.101020
Mallapaty S. India’s DNA COVID vaccine is a world first — more are coming. Nature. 2021;597:161-162. https://doi.org/10.1038/d41586-021-02385-x
Alberer M, Gnad-Vogt U, Hong HS, Mehr KT, Backert L, Finak G, Gottardo R, Bica MA, Garofano A, Koch SD, Fotin-Mleczek M, Hoerr I, Clemens R, von Sonnenburg F. Safety and immunogenicity of a mRNA rabies vaccine in healthy adults: an open-label, non-randomised, prospective, first-in-human phase 1 clinical trial. Lancet. 2017;390:1511-1520. https://doi.org/10.1016/S0140-6736(17)31665-3
Borgoyakova MB, Karpenko LI, Rudometov AP, Shanshin DV, Isaeva AA, Nesmeyanova VS, Volkova NV, Belenkaya SV, Murashkin DE, Shcherbakov DN, Volosnikova EA, Starostina EV, Orlova LA, Danilchenko NV, Zaikovskaya AV, Pyankov OV, Ilyichev AA. Immunogenic properties of the DNA construct encoding the receptor-binding domain of the SARS-CoV-2 spike protein. Mol. Biol. 2021;55(6):889-898. https://doi.org/10.1134/S0026893321050046
Gorchakov AA, Kulemzin SV, Guselnikov SV, Baranov KO, Belovezhets TN, Mechetina LV, Volkova OY, Najakshin AM, Chikaev NA, Chikaev AN, Solodkov PP, Larichev VF, Gulyaeva MA, Markhaev AG, Kononova YV, Alekseyev AY, Shestopalov AM, Yusubalieva GM, Klypa TV, Ivanov AV, Valuev-Elliston VT, Baklaushev VP, Taranin AV. Isolation of a panel of ultra-potent human antibodies neutralizing SARS-CoV-2 and viral variants of concern. Cell Discov. 2021;7(1):96. https://doi.org/10.1038/s41421-021-00340-8
Merkuleva IA, Shcherbakov DN, Borgoyakova MB, Shanshin DV, Rudometov AP, Karpenko LI, Belenkaya SV, Isaeva AA, Nesmeyanova VS, Kazachinskaia EI, Volosnikova EA, Esina TI, Zaykovskaya AV, Pyankov OV, Borisevich SS, Shelemba AA, Chikaev AN, Ilyichev AA. Comparative immunogenicity of the recombinant receptor-binding domain of protein S SARS-CoV-2 obtained in prokaryotic and mammalian expression systems. Vaccines (Basel). 2022;10(1):96. https://doi.org/10.3390/vaccines10010096
Rudometova NB, Shcherbakov DN, Karpenko LI. Generation and characterization of SARS-CoV-2 pseudoviruses. Med. Akad. Zh. 2022;22(2):249-253. Russian. https://doi.org/10.17816/MAJ108600
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Byulleten’ Eksperimental’noi Biologii i Meditsiny, Vol. 176, No. 12, pp. 751-756, December, 2023
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kisakov, D.N., Kisakova, L.A., Sharabrin, S.V. et al. Delivery of Experimental mRNA Vaccine Encoding the RBD of SARS-CoV-2 by Jet Injection. Bull Exp Biol Med 176, 776–780 (2024). https://doi.org/10.1007/s10517-024-06107-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10517-024-06107-x