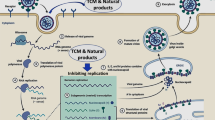
Abstract
Coronavirus disease 2019 (COVID-19) is an acute infectious respiratory disease that has been prevalent since December 2019. Chinese medicine (CM) has demonstrated its unique advantages in the fight against COVID-19 in the areas of disease prevention, improvement of clinical symptoms, and control of disease progression. This review summarized the relevant material components of CM in the treatment of COVID-19 by searching the relevant literature and reports on CM in the treatment of COVID-19 and combining with the physiological and pathological characteristics of the novel coronavirus. On the basis of sorting out experimental methods in vivo and in vitro, the mechanism of herb action was further clarified in terms of inhibiting virus invasion and replication and improving related complications. The aim of the article is to explore the strengths and characteristics of CM in the treatment of COVID-19, and to provide a basis for the research and scientific, standardized treatment of COVID-19 with CM.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.References
Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727–733.
Thompson MG, Burgess JL, Naleway AL, et al. Prevention and attenuation of COVID-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med 2021;385:320–329.
Yan J, Liu A, Huang J, et al. Research progress of drug treatment in novel coronavirus pneumonia. AAPS Pharm Sci Tech 2020;21:130–136.
Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res 2020;7:4.
Shi K, Liu Y, Zhang Q, et al. Severe type of COVID-19: pathogenesis, warning indicators and treatment. Chin J Integr Med 2022;28:3–11.
Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J 2020;133:1087–1095.
An X, Xu X, Xiao M, et al. Efficacy of Jinhua Qinggan Granules combined with Western medicine in the treatment of confirmed and suspected COVID-19: a randomized controlled trial. Front Med (Lausanne) 2021;8:728055.
Liu Z, Li X, Gou C, et al. Effect of Jinhua Qinggan Granules on novel coronavirus pneumonia in patients. J Tradit Chin Med 2020;40:467–72.
Gajewski A, Kosmider A, Nowacka A, et al. Potential of herbal products in prevention and treatment of COVID-19. Literature review. Biomed Pharmacother 2021;143:112150.
Zhu Y, Han Q, Wang L, et al. Jinhua Qinggan Granule attenuates acute lung injury by promotion of neutrophil apoptosis and inhibition of TLR4/MyD88/NF- κ B pathway. J Ethnopharmacol 2023;301:115763.
Zeng Y, Chen Y, He T, et al. Identification of components in vitro and plasma of Jinhua Qinggan Granules and preliminary pharmacokinetics analysis. ACTA Pharm Sin 2022;57:446–452.
Xiang MF, Jin CT, Sun LH, et al. Efficacy and potential mechanisms of Chinese herbal compounds in coronavirus disease 2019: advances of laboratory and clinical studies. Chin Med 2021;16:130.
Ren Y, Yin ZH, Dai JX, et al. Evidence-based complementary and alternative medicine exploring active components and mechanism of Jinhua Qinggan Granules in treatment of COVID-19 based on virus-host interaction. Nat Prod Commun 2020;15:1–11.
Sun XH, Zhang S, Yang Z, et al. Efficacy and safety of Lianhua Qingwen for patients with COVID-19: a systematic review and meta-analysis. Chin J Integr Med 2022;28:650–660.
Chen X, Wu Y, Chen C, et al. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen Capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm Sin B 2021;11:222–236.
Zhao P, Yang HZ, Lv HY, et al. Efficacy of Lianhuaqingwen Capsule compared with oseltamivir for influenza A virus infection: a meta-analysis of randomized, controlled trials. Altern Ther Health Med 2014;20:25–30.
Xia QD, Xun Y, Lu JL, et al. Network pharmacology and molecular docking analyses on Lianhua Qingwen Capsule indicate AKT1 is a potential target to treat and prevent COVID-19. Cell Prolif 2020;53:e12949.
Zhang Y, Wang J, Liu YM, et al. Analysis of the efficacy and mechanism of action of Xuebijing Injection on ARDS using meta-analysis and network pharmacology. Biomed Res Int 2021;2021:8824059.
Li C, Wang P, Li M, et al. The current evidence for the treatment of sepsis with Xuebijing Injection: bioactive constituents, findings of clinical studies and potential mechanisms. J Ethnopharmacol 2021;265:113301.
Sun Z, Zuo L, Sun T, et al. Chemical profiling and quantification of Xuebijing Injection, a systematic quality control strategy using UHPLC-Q exactive hybrid quadrupole-orbitrap high-resolution mass spectrometry. Sci Rep 2017;7:16921.
Ouyang HZ, He J. Simultaneous determination of nine constituents of Xuebijing Injection in rat plasma and their pharmacokinetics by LC-MS/MS. China J Chin Mater Med (Chin) 2018;43:3553–3561.
He TM, Duan CC, Li XF, et al. Potential mechanism of Xuebijing Injection in treatment of coronavirus pneumonia based on network pharmacology and molecular docking. Chin J Mod Appl Pharm (Chin) 2020;37:398–405.
Xing Y, Hua YR, Shang J, et al. Traditional Chinese medicine network pharmacology study on exploring the mechanism of Xuebijing Injection in the treatment of coronavirus disease 2019. Chin J Nat Med 2020;18:941–951.
Zheng WJ, Yan Q, Ni YS, et al. Examining the effector mechanisms of Xuebijing Injection on COVID-19 based on network pharmacology. BioData Min 2020;13:17.
Pu W, Zhang H, Wang M, et al. Superior stability of hydroxysafflor yellow A in Xuebijing Injection and the associated mechanism. Molecules 2017;22:2129.
Shi N, Liu B, Liang N, et al. Association between early treatment with Qingfei Paidu Decoction and favorable clinical outcomes in patients with COVID-19: a retrospective multicenter cohort study. Pharmacol Res 2020;161:105290.
Fan Y, Wang Y, Ma Y, et al. Analysis on composition mecharism of Qingfei Paidu Decoction from pathogenesis of cold pestilence of COVlD-19. Chin J Exp Tradit Med Form 2020;26:1–5.
Liu W, Huang J, Zhang F, et al. Comprehensive profiling and characterization of the absorbed components and metabolites in mice serum and tissues following oral administration of Qing-Fei-Pai-Du Decoction by UHPLC-Q-Exactive-Orbitrap HRMS. Chin J Nat Med 2021;19:305–320.
Xu D, Xu Y, Wang Z, et al. To study the mechanism of Qingfei Paidu Decoction in the treatment of novel coronavirus pneumonia based on network pharmacology. Pharmacol Clin Chin Mater Med (Chin) 2020;36:26–32.
Xu F, Hou T, Shen A, et al. Mechanism deconvolution of Qing Fei Pai Du Decoction for treatment of coronavirus disease 2019 (COVID-19) by label-free integrative pharmacology assays. J Ethnopharmacol 2021;280:114488.
Fu L, Shao S, Feng Y, et al. Mechanism of microbial metabolite leupeptin in the treatment of COVID-19 by traditional Chinese medicine herbs. mBio 2021;12:e0222021.
Zhao J, Tian S, Lu D, et al. Systems pharmacological study illustrates the immune regulation, anti-infection, antiinflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du Decoction in the treatment of COVID-19. Phytomedicine 2021;85:153315.
Wu H, Wang J, Yang Y, et al. To explore the mechanism of Qingfei Paidu Decoction against COVID-19 based on network pharmacology and molecular docking technology. Acta Pharm Sin B 2020;55:374–383.
Liu J, Yang W, Liu Y, et al. Combination of Hua Shi Bai Du Granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): a single-center, open-label, randomized controlled trial. Phytomedicine 2021;91:153671.
Xia KY, Zhao Z, Shah T, et al. Composition, clinical efficiency, and mechanism of NHC-approved “three Chinese medicines and three Chinese recipes” for COVID-19 treatment. Front Pharmacol 2022;12:781090.
Wei WL, Wu SF, Li HJ, et al. Chemical profiling of Huashi Baidu Prescription, an effective anti-COVID-19 TCM formula, by UPLC-Q-TOF/MS. Chin J Nat Med 2021;19:473–480.
Tao Q, Du J, Li X, et al. Network pharmacology and molecular docking analysis on molecular targets and mechanisms of Huashi Baidu Formula in the treatment of COVID-19. Drug Dev Ind Pharm 2020;46:1345–1353.
Xie YZ, Zhong CT, Ji SL, et al. The molecular mechanism of Huashi Baidu Decoction in the treatment of COVID-19-based on network pharmacology and molecular docking technology. Modern Tradit Chin Med Mater Med World Sci Technol (Chin) 2021;23:1048–1062.
Xiong WZ, Wang G, Du J, et al. Efficacy of herbal medicine (Xuanfei Baidu Decoction) combined with conventional drug in treating COVID-19: a pilot randomized clinical trial. Integr Med Res 2020;9:100489.
Zhao J, Guo D, Fan M, et al. Efficacy and safety of Xuanfei Baidu Granules for treating COVID-19: a protocol for systematic review and meta-analysis. Medicine 2021;100:e25653.
Wang Z, Zhang J, Zhan J, et al. Screening out antiinflammatory or anti-viral targets in Xuanfei Baidu Tang through a new technique of reverse finding target. Bioorg Chem 2021;116:105274.
Wang H, Song HX, Wang DF, et al. To explore the potential mechanism of Xuanfei-Baidu Decoction in the treatment of COVID-19 based on network pharmacology and molecular docking. Hainan Med J (Chin) 2020;26:1361–1372.
Zhang X, Kou YN, Yao SC, et al. Component identification and analysis in vivo of Sanhan Huashi Formula. China J Chin Mater Med (Chin) 2023;48:2126–2143.
Li L, Yu X, Xie D, et al. Influence of traditional Chinese medicines on the in vivo metabolism of lopinavir/ritonavir based on UHPLC-MS/MS analysis. J Pharm Anal 2022;12:270–277.
Ma Q, Wang Z, Chen R, et al. Effect of Jinzhen Granule on two coronaviruses: the novel SARS-CoV-2 and the HCoV-229E and the evidences for their mechanisms of action. Phytomedicine 2022;95:153874.
Xie R, Lin Z, Zhong C, et al. Deciphering the potential anti-COVID-19 active ingredients in Andrographis paniculata (Burm. F.) Nees by combination of network pharmacology, molecular docking, and molecular dynamics. RSC Adv 2021;11:36511–36517.
Pan HD, Yao XJ, Wang WY, et al. Network pharmacological approach for elucidating the mechanisms of traditional Chinese medicine in treating COVID-19 patients. Pharmacol Res 2020;159:105043.
Tang WF, Tsai HP, Chang YH, et al. Perilla (Perilla frutescens) leaf extract inhibits SARS-CoV-2 via direct virus inactivation. Biomed J 2021;44:293–303.
Kim TY, Jeon S, Jang Y, et al. Platycodin D, a natural component of Platycodon grandiflorum, prevents both lysosome- and TMPRSS2-driven SARS-CoV-2 infection by hindering membrane fusion. Exp Mol Med 2021;53:956–972.
Xu H, Li J, Song S, et al. Effective inhibition of coronavirus replication by Polygonum cuspidatum. Front Biosci (Landmark Ed) 2021;26:789–798.
Shanmugarajan D, P P, Kumar BRP, et al. Curcumin to inhibit binding of spike glycoprotein to ACE2 receptors: computational modelling, simulations, and ADMET studies to explore curcuminoids against novel SARS-CoV-2 targets. RSC Adv 2020;10:31385–31399.
Su HX, Yao S, Zhao WF, et al. Anti-SARS-CoV-2 activities in vitro of Shuanghuanglian preparations and bioactive ingredients. Acta Pharmacol Sin 2020;41:1167–1677.
Ryu YB, Jeong HJ, Kim JH, et al. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CL(pro) inhibition. Bioorgan Med Chem 2010;18:7940–7947.
Rakshit G, Dagur P, Satpathy S, et al. Flavonoids as potential therapeutics against novel coronavirus disease-2019 (nCOVID-19). J Biomol Struct Dyn 2022;40:6989–7001.
Bahadur Gurung A, Ajmal Ali M, Lee J, et al. Identification of SARS-CoV-2 inhibitors from extracts of Houttuynia cordata Thunb. Saudi J Biol Sci 2021;28:7517–7527.
Konishi K, Yamaji T, Sakuma C, et al. Whole-genome sequencing of vero E6 (VERO C1008) and comparative analysis of four vero cell sublines. Front Genet 2022;13:801382.
Hao S, Ning K, Kuz CA, et al. Long-term modeling SARS-CoV-2 infection of in vitro cultured polarized human airway epithelium. mBio 2020;11:e02852–20.
Sasaki M, Kishimoto M, Itakura Y, et al. Air-liquid interphase culture confers SARS-CoV-2 susceptibility to A549 alveolar epithelial cells. Biochem Biophys Res Commun 2021;577:146–151.
Sun SH, Chen Q, Gu HJ, et al. A mouse model of SARS-CoV-2 infection and pathogenesis. Cell Host Microbe 2020;28:124–33.e4.
Sun J, Zhuang Z, Zheng J, et al. Generation of a broadly useful model for COVID-19 pathogenesis, vaccination, and treatment. Cell 2020;182:734–43.e5.
Cui XR, Guo YH, Liu QQ. Cangma Huadu Granules, a new drug with great potential to treat coronavirus and influenza infections, exert its efficacy through anti-inflammatory and immune regulation. J Ethnopharmacol 2022;287:114965.
Gu J, Han B, Wang J. COVID-19: Gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology 2020;158:1518–1519.
Yan H, Lu J, Wang J, et al. Prevention of cyclophosphamide-induced immunosuppression in mice with traditional Chinese medicine Xuanfei Baidu Decoction. Front Pharmacol 2021;12:730567.
Wang Y, Sang X, Shao R, et al. Xuanfei Baidu Decoction protects against macrophages induced inflammation and pulmonary fibrosis via inhibiting IL-6/STAT3 signaling pathway. J Ethnopharmacol 2022;283:114701.
Geng ZH, Bao L, Guo S, et al. To establish and evaluate a mouse model of human coronavirus 229E cold-dampness epidemic virus attacking the lung. Chin J Comparat Med (Chin) 2022;32:3–12,67.
Zhu SM, Ning WM, Dong MG, et al. Establishment and evaluation of a mouse model of cold and damp epidemic of COVID-19. Chin J Comparat Med (Chin) 2022;32:62–68.
Pang B, Zhang JS, Guo S, et al. To establish and evaluate a mouse model of human coronavirus 229E damp-heat epidemic virus attacking lung disease. Chin J Pharmacovigil (Chin) 2023;20:40–45.
Harcourt J, Tamin A, Lu X, et al. Severe acute respiratory syndrome coronavirus 2 from patient with coronavirus disease, United States. Emerg Infect Dis 2020;26:1266–1273.
Barreto-Vieira DF, da Silva MAN, Garcia CC, et al. Morphology and morphogenesis of SARS-CoV-2 in Vero-E6 cells. Mem Inst Oswaldo Cruz 2021;116:e200443.
Runfeng L, Yunlong H, Jicheng H, et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol Res 2020;156:104761.
Gao J, Cao C, Shi M, et al. Kaempferol inhibits SARS-CoV-2 invasion by impairing heptad repeats-mediated viral fusion. Phytomedicine 2023;118:154942.
Guo S, Zhao R, Geng Z, et al. The Mechanism of Qingkailing Injection aganist coronavirus pneumonia. Chin J Pharmacovigil (Chin) 2021;18:1111–1116.
Gupta A, Madhavan MV, Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–1032.
Li L, Wu Y, Wang J, et al. Potential treatment of COVID-19 with traditional Chinese medicine: what herbs can help win the battle with SARS-CoV-2? Engineering 2022;19:139–152.
Huang YF, Bai C, He F, et al. Review on the potential action mechanisms of Chinese medicines in treating coronavirus disease 2019 (COVID-19). Pharmacol Res 2020;158:104939.
Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–1034.
Bilaloglu S, Aphinyanaphongs Y, Jones S, et al. Thrombosis in hospitalized patients with COVID-19 in a New York City Health System. JAMA 2020;324:799–801.
Liu F, Liu F, Wang L. COVID-19 and cardiovascular diseases. J Mol Cell Biol 2021;13:161–167.
Faheem, Kumar BK, Sekhar K, et al. Druggable targets of SARS-CoV-2 and treatment opportunities for COVID-19. Bioorg Chem 2020;104:104269.
Buzas EI. The roles of extracellular vesicles in the immune system. Nat Rev Immunol 2023;23:236–250.
Hong L, He M, Li S, et al. Predicting for anti-(mutant) SARS-CoV-2 and anti-inflammation compounds of Lianhua Qingwen Capsules in treating COVID-19. Chin Med 2022;17:84.
Niu W, Wu F, Cui H, et al. Network pharmacology analysis to identify phytochemicals in traditional Chinese medicines that may regulate ACE2 for the treatment of COVID-19. Evid Based Complement Alternat Med 2020;2020:7493281.
Jacques FH, Apedaile E. Immunopathogenesis of COVID-19: summary and possible interventions. Front Immunol 2020;11:564925.
Schultze JL, Aschenbrenner AC. COVID-19 and the human innate immune system. Cell 2021;184:1671–1692.
Anka AU, Tahir MI, Abubakar SD, et al. Coronavirus disease 2019 (COVID-19): an overview of the immunopathology, serological diagnosis and management. Scand J Immunol 2021;93:e12998.
Mazaleuskaya L, Veltrop R, Ikpeze N, et al. Protective role of toll-like receptor 3-induced type I interferon in murine coronavirus infection of macrophages. Viruses 2012;4:901–923.
Park A, Iwasaki A. Type I and type M interferons—induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe 2020;27:870–888.
Hur S. Double-stranded RNA sensors and modulators in innate immunity. Annu Rev Immunol 2019;37:349–375.
Mulchandani R, Lyngdoh T, Kakkar AK. Deciphering the COVID-19 cytokine storm: systematic review and meta-analysis. Eur J Clin Invest 2021;51:e13429.
Tharmarajah E, Buazon A, Patel V, et al. IL-6 inhibition in the treatment of COVID-19: a meta-analysis and meta-regression. J Infect 2021;82:178–185.
Shi Y, Wang Y, Shao C, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ 2020;27:1451–1454.
Tian S, Zheng N, Zu X, et al. Integrated hepatic single-cell RNA sequencing and untargeted metabolomics reveals the immune and metabolic modulation of Qing-Fei-Pai-Du Decoction in mice with coronavirus-induced pneumonia. Phytomedicine 2022;97:153922.
Yang R, Liu H, Bai C, et al. Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against coronavirus disease 2019 (COVID-19): in silico and experimental study. Pharmacol Res 2020;157:104820.
Ren W, Ma Y, Wang R, et al. Research advance on Qingfei Paidu Decoction in prescription principle, mechanism analysis and clinical application. Front Pharmacol 2020;11:589714.
Dai Y, Qiang W, Gui Y, et al. A large-scale transcriptional study reveals inhibition of COVID-19 related cytokine storm by traditional Chinese medicines. Sci Bull (Beijing) 2021;66:884–888.
Xiong L, Cao J, Yang X, et al. Exploring the mechanism of action of Xuanfei Baidu Granule (XFBD) in the treatment of COVID-19 based on molecular docking and molecular dynamics. Front Cell Infect Microbiol 2022;12:965273.
Wang Y, Wang X, Li Y, et al. Xuanfei Baidu Decoction reduces acute lung injury by regulating infiltration of neutrophils and macrophages via PD-1/IL17A pathway. Pharmacol Res 2022;176:106083.
Zhang X, Gao R, Zhou Z, et al. A network pharmacology based approach for predicting active ingredients and potential mechanism of Lianhuaqingwen Capsule in treating COVID-19. Int J Med Sci 2021;18:1866–1876.
Liang C, Hui N, Liu Y, et al. Insights into Forsythia Honeysuckle (Lianhuaqingwen) Capsules: a Chinese herbal medicine repurposed for COVID-19 pandemic. Phytomed Plus 2021;1:100027.
Chen X, Feng Y, Shen X, et al. Anti-sepsis protection of Xuebijing Injection is mediated by differential regulation of pro- and anti-inflammatory Th17 and T regulatory cells in a murine model of polymicrobial sepsis. J Ethnopharmacol 2018;211:358–365.
Jiang Y, Zou L, Liu S, et al. GC/MS-based metabonomics approach reveals effects of Xuebijing Injection in CLP induced septic rats. Biomed Pharmacother 2019;117:109163.
Li X, Wu D, Niu J, et al. Intestinal Flora: a pivotal role in investigation of traditional Chinese medicine. Am J Chin Med 2021;49:237–268.
Cyranoski D. Profile of a killer: the complex biology powering the coronavirus pandemic. Nature 2020;581:22–26.
Lamers MM, Beumer J, van der Vaart J, et al. SARS-CoV-2 productively infects human gut enterocytes. Science 2020;369:50–54.
Effenberger M, Grabherr F, Mayr L, et al. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut 2020;69:1543–1544.
Ma L, Zhao X, Liu T, et al. Xuanfei Baidu Decoction attenuates intestinal disorders by modulating NF- κ B pathway, regulating T cell immunity and improving intestinal flora. Phytomedicine 2022;101:154100.
Wu G, Zhang W, Zheng N, et al. Integrated microbiome and metabolome analysis reveals the potential therapeutic mechanism of Qing-Fei-Pai-Du Decoction in mice with coronavirus-induced pneumonia. Front Cell Infect Microbiol 2022;12:950983.
Lyu M, Wang YF, Fan GW, et al. Balancing herbal medicine and functional food for prevention and treatment of cardiometabolic diseases through modulating gut microbiota. Front Microbiol 2017;8:2146.
Oudit GY, Wang KM, Viveiros A, et al. Angiotensin-converting enzyme 2—at the heart of the COVID-19 pandemic. Cell 2023;186:906–922.
Parker MFL, Blecha J, Rosenberg O, et al. Cyclic 68Galabeled peptides for specific detection of human angiotensin-converting enzyme 2. J Nucl Med 2021;62:1631–1637.
Zhou W, Chen Z, Fang Z, et al. Network analysis for elucidating the mechanisms of Shenfu Injection in preventing and treating COVID-19 combined with heart failure. Comput Biol Med 2022;148:105845.
Xia Y, S D, Jiang S, et al. YiQiFuMai Lyophilized Injection attenuates particulate matter-induced acute lung injury in mice via TLR4-mTOR-autophagy pathway. Biomed Pharmacother 2018;108:906–913.
Xu Y, Chen K, Pan J, et al. Repurposing clinically approved drugs for COVID-19 treatment targeting SARS-CoV-2 papain-like protease. Int J Biol Macromol 2021;188:137–146.
Liu T, Guo Y, Zhao J, et al. Systems pharmacology and verification of Shenfuhuang Formula in zebrafish model reveal multi-scale treatment strategy for septic syndrome in COVID-19. Front Pharmacol 2020;11:584057.
Chen Y, Tong H, Zhang X, et al. Xuebijing Injection alleviates liver injury by inhibiting secretory function of Kupffer cells in heat stroke rats. J Tradit Chin Med 2013;33:243–249.
Ji M, Wang Y, Wang L, et al. Protective effect of Xuebijing Injection against acute lung injury induced by left ventricular ischemia/reperfusion in rabbits. Exp Ther Med 2016;12:51–58.
Zhang H, Wei L, Zhao G, et al. Protective effect of Xuebijing Injection on myocardial injury in patients with sepsis: a randomized clinical trial. J Tradit Chin Med 2016;36:706–710.
Liu X, Hu Z, Zhou B, et al. Chinese herbal preparation Xuebijing potently inhibits inflammasome activation in hepatocytes and ameliorates mouse liver ischemia-reperfusion injury. PLoS One 2015;10:e0131436.
Liu M, Gao Y, Yuan Y, et al. Efficacy and safety of integrated traditional Chinese and Western medicine for corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. Pharmacol Res 2020;158:104896.
Xiong X, Wang P, Su K, et al. Chinese herbal medicine for coronavirus disease 2019: a systematic review and meta-analysis. Pharmacol Res 2020;160:105056.
Li Q, Wang H, Li X, et al. The role played by traditional Chinese medicine in preventing and treating COVID-19 in China. Front Med 2020;14:681–688.
Xing D, Liu Z. Effectiveness and safety of traditional Chinese medicine in treating COVID-19: clinical evidence from China. Aging Dis 2021;12:1850–1856.
Ji X, Meng X, Zhu X, et al. Research and development of Chinese anti-COVID-19 drugs. Acta Pharm Sin B 2022;12:4271–4286.
Yang F, Zhang S, Pan W, et al. Signaling repurposable drug combinations against COVID-19 by developing the heterogeneous deep herb-graph method. Brief Bioinform 2022;23:1–21.
Qi X, Li B, Omarini AB, et al. Discovery of TCMs and derivatives against the main protease of SARS-CoV-2 via high throughput screening, ADMET analysis, and inhibition assay in vitro. J Mol Struct 2022;1268:133709.
Acknowledgements
We are grateful the support from the Key Laboratory of Dongzhimen Hospital, Beijing University of Chinese Medicine.
Author information
Authors and Affiliations
Contributions
Xu QQ, Fan XD and Yu DD designed, drafted and revised the manuscript. Cui HR guided the writing of the article. Dai QQ, Zhang XY and Zhong XY searched the databases. Zhao C provided methodological guidance. You LZ and Shang HC conceived and supervised the study. All authors agreed the final version of this article.
Corresponding author
Ethics declarations
The authors declare that they have no competing interests.
Additional information
Supported by the State Administration of Traditional Chinese Medicine (No. GZY-KJS-2021-055)
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Xu, Qq., Yu, Dd., Fan, Xd. et al. Chinese Medicine for Treatment of COVID-19: A Review of Potential Pharmacological Components and Mechanisms. Chin. J. Integr. Med. 31, 83–95 (2025). https://doi.org/10.1007/s11655-024-3909-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11655-024-3909-z