Abstract
The long-term effects of SARS-CoV-2 infection, and their determinants, are still unknown. This study aimed to assess symptoms one year after admission for COVID-19, according to the organ/system involved, and to identify factors. Cross-sectional study with retrospective data collection from March 2020 to February 2021. Inclusion criteria: aged ≥ 18 years and admitted for COVID-19. Exclusion criteria: death, not localized, refusal to participate, cognitive impairment or language barrier. A telephone survey was conducted on long COVID-related symptoms one year after hospital discharge. n = 486. The most frequent symptom groups were neurological (n = 225; 46.3%) and respiratory (n = 201; 41.4%). Multivariable analysis showed that a history of anxiety was significantly associated with psychiatric symptoms (ORa = 2.04, 95%CI = 1.02–4.06), fibromyalgia/chronic fatigue with general symptoms (ORa = 11.59, 95%CI = 1.47–9.34) and obesity with respiratory (ORa 1.90, 95%CI = 1.27–2.83) and musculoskeletal symptoms (ORa 1.96, 95%CI = 1.30–2.96). Male sex was associated with a significantly lower risk of neurological (ORa 0.64, 95%CI = 0.44–0.93), respiratory (ORa 0.45, 95%CI = 0.31–0.67), general (ORa 0.43, 95%CI = 0.29–0.63), psychiatric (ORa 0.34, 95%CI = 0.22–0.51), musculoskeletal (ORa 0.47, 95%CI = 0.32–0.70), dermatological (ORa 0.24, 95%CI = 0.14–0.42) and digestive (ORa 0.38, 95%CI = 0.20–0.73) symptoms. Advanced age (≥ 71 years) also had a protective effect against general (ORa 0.60, 95%CI = 0.39–0.95), psychiatric (ORa 0.39, 95%CI = 0.23–0.64), and dermatological (ORa 0.47, 95%CI = 0.24–0.92) symptoms. Patients admitted for SARS-CoV-2 infection frequently experience symptoms at one year, especially neurological and respiratory symptoms. Female sex, obesity, a history of anxiety and fibromyalgia/chronic fatigue were independent risk factors for presenting symptoms. Advanced age acted as a protective factor.
Similar content being viewed by others
Introduction
Between the end of 2019, when the first case was reported, until 10 March 2023, SAR-CoV-2 caused nearly 700 million confirmed infections worldwide and almost 7 million deaths1. Although its pathophysiology and clinical manifestations in the acute phase are already well known2, details on its long-term evolution, including the factors influencing it and its management, still need elucidation. This knowledge is of the utmost importance, since around 10–20% of infected patients still present symptoms or medical complications weeks or even months after infection3. In October 2021, the World Health Organization (WHO) defined long COVID as the presence of symptoms persisting for at least two months and at least three months after acute SARS-CoV-2 infection, which cannot be explained by an alternative diagnosis4. Long COVID, also called post-COVID condition or post-acute sequelae of COVID-19 (PASC), can affect any organ or system of the human body, including the central and peripheral nervous, cardiovascular, respiratory, and digestive systems5,6,7,8. In this line, a recent meta-analysis in 1.2 million patients who had suffered an acute symptomatic SARS-CoV-2 infection showed that about 6.2% still had symptoms compatible with long COVID three months after infection9. Its long-term association with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and dysautonomia, especially with postural orthostatic tachycardia syndrome, is also important10,11. Moreover, evidence points to its significant impact on patients’ quality of life, even a year after acute infection and regardless of the severity of the episode12.
Therefore, our study aimed to assess the presence of symptoms one year after admission for SARS-CoV-2 infection, according to the organ/system involved, and to identify the factors influencing the appearance of each symptom group.
Materials and methods
Study design, setting and participants
This cross-sectional study with retrospective data collection took place in the city of Castellón (Spain), in a referral hospital serving about 283,000 inhabitants, from March 2020 to February 2021. Patients over 18 years of age admitted to the infectious diseases unit for SARS-CoV-2 infection, confirmed by RT-PCR or antigenic test, were included. Patients were excluded if they died during admission or subsequent follow-up up to one year after the hospital discharge, could not be located at the time of the telephone interview, refused to participate in the study, had a relevant cognitive impairment at the time of the interview, or could not comfortably communicate in a language known to the researchers. The patients finally included in the study underwent a telephone survey in which they were asked directly about the presence or absence of the main Long-COVID-related symptoms (grouped by organ and systems, as presented in Table 1). There was also an open question about any other symptoms they had experienced, apart from those mentioned in the interview.
Variables
Electronic health records (Orion Clinic-Conselleria de Sanitat Universal i Salut Publica, Comunidad Valenciana, Spain) were reviewed retrospectively one year after discharge from hospital to collect explanatory variables, including: demographic data (sex, age); disease history [comorbidities including obesity, defined by body mass index ≥ 30 kg/m2, and age-adjusted Charlson comorbidity index (scale for assessing life expectancy at ten years, depending on the age at which it is assessed, and the subject’s comorbidities)]; clinical course (hospital stay, evolution to acute respiratory distress syndrome [ARDS], need for admission to the intensive care unit [ICU], type of respiratory support, inspiratory O2 fraction [FiO2] and Pa/FiO2 ratio on admission and extreme values during their stay); analytical parameters during hospital stay (values on admission and extreme values during admission of lymphocytes, C-reactive protein [CRP], ferritin, IL-6 [interleukin-6] and D-dimer); treatment (systemic corticotherapy during admission and total days of corticotherapy); and vaccination against SARS-CoV-2 after hospital admission (yes/no).
Participants who gave informed consent completed a telephone survey already described above, one year after admission, which constituted the primary outcome.
Statistical analysis
The statistical analysis was undertaken using SPSS software (version 23; IBM). First, a descriptive study of the sample was performed, expressing categorical variables as absolute or relative frequencies and quantitative variables as means (standard deviation, SD) or medians (interquartile range, IQR), depending on the normality of their distribution (only age showed a normal distribution). Next, the association between the different explanatory variables and outcomes was studied. Results were expressed by odds ratio (OR) and 95% confidence intervals (CI). Quantitative variables were dichotomized, using the 75th percentile as the cutoff value. The Bonferroni test was used to correct for multiple comparisons, so considering a statistical significance of p < 0.05 and the fact that 39 variables were included in the univariable analysis, only values of p < 0.0012 were considered statistically significant. Finally, a multivariable analysis was completed, using binary logistic regression, including in the model the variables that had been shown to have a statistically significant influence on the presence or absence of the different symptom clusters. The analysis was also adjusted for sex and age.
Ethics
The research ethics committee of the Castellón General University Hospital approved the study, which complied with the guidelines of the Spanish Agency of Medicines and Health Products. All participants gave their verbal informed consent. Patient confidentiality and personal data were protected according to Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of data.
Results
Study sample
After excluding 334 patients, a total of 486 patients were included (Fig. 1): 292 (60.1%) were men, and the mean age was 61 years (SD 14). Nearly a quarter (n = 111, 22.8%) were smokers or former smokers, 205 (44.2%) had a diagnosis of hypertension, and 153 (31.5%) were obese. The median age-adjusted Charslon comorbidity index score was 2 (IQR 1–3). With regard to the clinical characteristics of the index hospitalization, the median length of stay was 10 days (IQR 6–15), and 100 patients (20.6%) required ICU admission, with a median unit stay of 6 days (IQR 4–10). In addition, 193 patients (39.7%) presented ARDS; 93 (19.1%) required noninvasive mechanical ventilation, and 17 (3.5%) invasive. Regarding pharmacological treatment, 432 patients (88.9%) received systemic corticosteroids during admission (median duration 36 days, IQR 19–49). Following admission, 398 patients (81.9%) completed the SARS-CoV-2 vaccination regimen recommended at the time by the country’s health authorities. None had received any dose of the SARS-CoV-2 vaccine prior to hospital admission. Patient characteristics, including analytical variables are described in full in Tables 2 and 3.
Self-reported symptoms at one-year follow-up
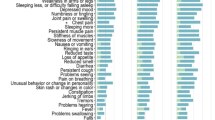
The most frequently self-reported symptoms at one year after admission for SARS-CoV-2 infection were mental fog (n = 177; 36.4%), asthenia (n = 167; 34.4%), dyspnea (n = 155; 31.9%), arthromyalgia (n = 147; 30.2%), and anxiety (n = 120; 24.7%) (Fig. 2). In terms of symptom groups (Table 1), neurological symptoms were the most frequent (n = 225, 46.3%), followed by respiratory (n = 201, 41.4%), general (n = 185, 38.1%), psychiatric (n = 168, 34.6%), and musculoskeletal (n = 149, 30.7%) symptoms. Only 144 patients (29.6%) reported having no symptoms at the time of the telephone interview (Fig. 3).
Association between explanatory variables and the presence of different symptom groups. Univariable analysis
A history of fibromyalgia/chronic fatigue was significantly associated with the presence of respiratory (OR = 8.19, 95%CI = 1.80-37.37), general (OR = 20.81, 95%CI = 2.68-161.41) and psychiatric (OR = 11.07, 95%CI = 2.42–50.55) symptoms. Obesity was the second most prominent risk factor, showing a statistical relationship with the presence of respiratory (OR = 2.00, 95%CI = 1.35–2.95) and musculoskeletal (OR = 2.01, 95%CI = 1.34–3.01) symptoms. Psychiatric symptoms were also associated with a history of anxiety (OR = 3.72, 95%CI = 2.09–6.64) and depression (OR = 3.47, 95%CI = 1.55–7.76).
In contrast, male sex showed a protective effect against respiratory (OR = 0.42, 95%CI = 0.29–0.60), general (OR = 0.40, 95%CI = 0.28–0.58), psychiatric (OR = 0.31, 95%CI = 0.21–0.46), musculoskeletal (OR = 0.46, 95%CI = 0.31–0.68) and dermatological (OR = 0.26, 95%CI = 0.15–0.44) symptoms (Table 2).
As for quantitative variables, advanced age (≥ 71 years) showed a protective effect against the presence of psychiatric symptoms at one year (OR = 0.44, 95%CI = 0.27–0.70). No other statistically significant associations were observed (Table 3).
Association between explanatory variables and the presence of different symptom groups. Multivariable analysis
A history of anxiety was significantly and independently associated with the presence of psychiatric symptoms at one year (ORa = 2.04, 95%CI = 1.02–4.06). Fibromyalgia/chronic fatigue was associated with the persistence of general symptoms (ORa = 11.59, 95%CI = 1.47–9.34), and obesity with respiratory (ORa = 1.90, 95%CI = 1.27–2.83) and musculoskeletal symptoms (ORa = 1.96, 95%CI = 1.30–2.96).
In contrast, male sex was associated with a lower presence of neurological (ORa = 0.64, 95%CI = 0.44–0.93), respiratory (ORa = 0.45, 95%CI = 0.31–0.67), general (ORa = 0.43, 95%CI = 0.29–0.63), psychiatric (ORa = 0.34, 95%CI = 0.22–0.51), musculoskeletal (ORa = 0.47; 95%CI = 0.32–0.70), dermatological (ORa = 0.24, 95%CI = 0.14–0.42), and digestive (ORa = 0.38, 95%CI = 0.20–0.73) symptoms. Advanced age (≥ 71 years) also had a protective effect against presenting general (ORa = 0.60, 95%CI = 0.39–0.95), psychiatric (ORa = 0.39, 95%CI = 0.23–0.64), and dermatological (ORa = 0.47, 95%CI = 0.24–0.92) symptoms (Table 4).
Discussion
Our study followed up on cohort of adult patients, mainly made up of men with an average age of around 60 years of age and a modest burden of comorbidity, who had been admitted for SARS-CoV-2 and had not been vaccinated against at the time of acute infection. Just under half developed ARDS, and practically all of them were treated with systemic corticosteroids. A year after discharge, they frequently showed symptoms that they attributed to COVID-19. Only 29.6% were completely asymptomatic, a remarkable figure although still somewhat higher than reports from similar prospective studies with one-year follow-up in samples that included mild cases not requiring hospital admission13,14. Those results are particularly striking considering that the estimated incidence of long COVID in hospitalized patients is 50–70%, compared to 10–30% in community cases15,16. However, this could be explained by differences between the populations studied, for example due to selection biases in studies analyzing outpatients who needed follow-up care during their recovery from the acute infection and thus may have been more prone to long-term symptoms.
In keeping with other studies, our data show that both neurological and respiratory symptoms were particularly frequent17,18, and generally and psychiatric symptoms somewhat less so, as described elsewhere19. Similarly, an international observational study of 3762 people from 56 different countries reported that, six months after infection, 65.2% of patients had at least one symptom17. Asthenia, weakness on exertion, and cognitive impairment were the most frequent17.
Multiple theories exist about the pathophysiology of long COVID, involving endothelial damage, immune dysregulation and autoimmunity phenomena, thrombotic phenomena, inflammation, intestinal dysbiosis, and even viral persistence in certain reservoirs20. Less is known about which socio-epidemiological, clinical, or analytical factors influence its appearance, much less which of these parameters are at play in different symptom groups. According to our results, a history of anxiety, fibromyalgia/chronic fatigue, obesity, and above all female sex behave as clear independent risk factors for the presence of symptoms after SARS-CoV-2 infection. Other published cohorts have also demonstrated a clear association between female sex and long COVID as well as poorer long-term quality of life21,22,23. Indeed, Subramanian et al. included more than 480,000 patients, observing that female sex, obesity, and a history of fibromyalgia or anxiety were independent risk factors for developing long COVID24. The risk of long COVID attributable to female sex has its counterpoint in the fact that men are at higher risk of death from COVID-1925. Thus, among patients at high risk for an adverse outcome, data show that men are more likely to die, and women to experience persistent symptoms. Furthermore, as observed in our analysis and also in the study by Cai et al., the age range in which the probability of suffering symptoms would increase significantly is around 40–60 years21.
Of note, the severity of the infection, as reflected by the degree of respiratory failure and type of respiratory support required, the inflammatory laboratory values, and the need for ICU admission, did not seem influence the appearance of any of the symptom groups at one year. Other authors have reported similar results, associating long COVID with a specific clinical profile (mainly female sex, obesity, and a history of fibromyalgia/chronic fatigue syndrome) rather than with the severity of the acute infection12. However, there are conflicting reports on this topic, with some studies finding that the severity of the acute infection was an independent determinant for long COVID21.
Results should be interpreted in light of the study period and its implications. First of all, our sample comprises people admitted to hospital in the first year of the pandemic, which suggests that the vast majority were infected by the Delta variant of SARS-CoV-2. This variant is associated with a higher incidence of long COVID than Omicron, which became the dominant variant later on21,26. Secondly, none of our patients had been vaccinated before hospital admission, and prior immunization has been shown to reduce the subsequent incidence of long COVID27,28,29. This consideration is relevant when comparing our results with those of other cohorts, since most studies include a high percentage of previously vaccinated patients. However, we consider that these points constitute a strength of the study, since our (unvaccinated) sample was infected with the SARS-CoV-2 variant that probably generates the most persistent long-term symptoms, making a study of the risk factors that influence long COVID in this population particularly interesting. Furthermore, unlike other studies, we not only analyzed the presence of symptoms by symptom groups but also looked at the potential relationship with poorly studied factors, such as analytical values during admission, the type of respiratory support used, and the medical treatment received.
That said, the study also has some limitations, beginning with its retrospective nature and the lack of a control group who did not require hospital admission or who were not infected with SARS-CoV-2. Another limitation, common to all the studies performed on this topic, is the lack of a baseline symptom survey prior to infection by SARS-CoV-2 with which to compare post-COVID symptoms, enabling a valid attribution of symptoms to long COVID. In addition, due to the considerable length of the questionnaire and the fact that all questions are answered with yes or no, some respondents may tend to answer yes or no to all questions [30]. Finally, as in most similar studies already published, the absence of a control group that has not been infected with SARS-CoV-2 is a notable limitation.
Conclusions
According to our results, patients admitted for SARS-CoV-2 infection during the first year of the pandemic reported the presence of symptoms related to the infection one year after hospitalization. Neurological and respiratory symptoms were the most frequent, with female sex, obesity, a history of anxiety and fibromyalgia/chronic fatigue standing out as independent risk factors. More studies are needed about the epidemiology, pathophysiology, and possible treatments for long COVID.
Data availability
Access to any other data is not facilitated since all relevant data is contained within the article.
Abbreviations
- OR:
-
Odds ratio
- WHO:
-
World Health Organization
- PASC:
-
Post-acute sequelae of COVID-19
- ME/CFS:
-
Encephalomyelitis/chronic fatigue syndrome
- ARDS:
-
Acute respiratory distress syndrome
- ICU:
-
Intensive unit care
- FiO2:
-
Inspiratory O2 fraction
- PaO2:
-
Partial pressure of oxygen
- RCP:
-
C-reactive protein
- IL-6:
-
Interleukin-6
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- CI:
-
Confidence interval
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- CPAP:
-
Continuous positive airway pressure
References
Coronavirus Resource Center. (2023). https://coronavirus.jhu.edu/map.html (accedido Enero 11, 2024).
Gandhi, R. T., Lynch, J. B. & Del Rio, C. Mild or moderate Covid-19. N Engl. J. Med. 383 (18), 1757–1766. https://doi.org/10.1056/NEJMcp2009249 (2020). Epub 2020 Apr 24.
Hanson, S. W. et al. A global systematic analysis of the occurrence, severity, and recovery pattern of long COVID in 2020 and 2021. medRxiv [Preprint]. (2022). https://doi.org/10.1101/2022.05.26.22275532
World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus. (2021). https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (Accedido Enero 11, 2024).
Logue, J. K. et al. Sequelae in adults at 6 months after COVID-19 infection. JAMA Netw. Open. 4, e210830. https://doi.org/10.1001/jamanetworkopen.2021.0830 (2021).
Sigfrid, L. et al. Long covid in adults discharged from UK hospitals after Covid-19: a prospective, multicentre cohort study using the ISARIC WHO clinical characterisation protocol. Lancet Reg. Health Eur. 8, 100186. https://doi.org/10.1016/j.lanepe.2021.100186 (2021).
Huang, C. et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 397, 220–232. https://doi.org/10.1016/S0140-6736(20)32656-8 (2021).
Huang, L. et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 10, 863–876. https://doi.org/10.1016/S2213-2600(22)00126-6 (2022).
Global Burden of Disease Long COVID Collaborators et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. 328:1604–15. doi: (2022). https://doi.org/10.1001/jama.2022.18931
Kedor, C. et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. ;13(1):5104. doi: (2022). https://doi.org/10.1038/s41467-022-32507-6. (Erratum in: Nat Commun. 2022;13(1):6009).
Larsen, N. W. et al. Characterization of autonomic symptom burden in long COVID: a global survey of 2,314 adults. Front. Neurol. 13, 1012668. https://doi.org/10.3389/fneur.2022.1012668 (2022).
Pérez Catalán, I. et al. One-year quality of life among post-hospitalization COVID-19 patients. Front. Public. Health. 11, 1236527. https://doi.org/10.3389/fpubh.2023.1236527 (2023).
Salmon, D. et al. Long COVID patients continue to experience significant symptoms at 12 months and factors associated with improvement: a prospective cohort study in France (PERSICOR). Int. J. Infect. Dis. https://doi.org/10.1016/j.ijid.2023.11.038 (2023).
Tran, V. T., Porcher, R., Pane, I. & Ravaud, P. Course of post COVID-19 disease symptoms over time in the ComPaRe long COVID prospective e-cohort. Nat. Commun. 13 (1), 1812. https://doi.org/10.1038/s41467-022-29513-z (2022).
Bull-Otterson, L. Post–COVID conditions among adult COVID-19 survivors aged 18–64 and ≥ 65 years — United States, March 2020–November 2021. MMWR Morb Mortal. Wkly. Rep. 71, 713 (2022).
Ceban, F. et al. Fatigue and cognitive impairment in Post-COVID-19 syndrome: a systematic review and meta-analysis. Brain Behav. Immun. 101, 93–135. https://doi.org/10.1016/j.bbi.2021.12.020 (2022).
Davis, H. E. et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 38, 101019. https://doi.org/10.1016/j.eclinm.2021.101019 (2021).
Spudich, S. & Nath, A. Nervous system consequences of COVID-19. Science. 375(6578), 267–269 (2022). https://doi.org/10.1126/science.abm2052.
Lau, B. et al. Physical and mental health disability associated with long-COVID: baseline results from a US nationwide cohort. medRxiv. https://doi.org/10.1016/j.amjmed.2023.08.009 (2022).
Davis, H. E., McCorkell, L., Vogel, J. M., Topol, E. J. & Long, C. O. V. I. D. Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 21 (3), 133–146. https://doi.org/10.1038/s41579-022-00846-2 (2023).
Cai, J. et al. A one-year follow-up study of systematic impact of long COVID symptoms among patients post SARS-CoV-2 omicron variants infection in Shanghai, China. Emerg. Microbes Infect. 12 (2), 2220578. https://doi.org/10.1080/22221751.2023.2220578 (2023).
Zhang, L. et al. Undiagnosed long COVID-19 in China among non-vaccinated individuals: identifying persistent symptoms and impacts on patients’ Health-Related Quality of Life. J. Epidemiol. Glob Health. 12 (4), 560–571. https://doi.org/10.1007/s44197-022-00079-9 (2022).
Anastasio, F. et al. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur. Respir J. 58 (3), 2004015. https://doi.org/10.1183/13993003.04015-2020 (2021).
Subramanian, A. et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 28 (8), 1706–1714. https://doi.org/10.1038/s41591-022-01909-w (2022).
Dessie, Z. G. & Zewotir, T. Mortality-related risk factors of COVID-19: a systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 21 (1), 855. https://doi.org/10.1186/s12879-021-06536-3 (2021).
Wu, Q. et al. Follow-up of patients with COVID-19 by the Delta variant after hospital discharge in Guangzhou, Guandong, China. Rev. Inst. Med. Trop. Sao Paulo. 64, e31. https://doi.org/10.1590/S1678-9946202264031 (2022).
Ayoubkhani, D. et al. Risk of long COVID in people infected with severe Acute Respiratory Syndrome Coronavirus 2 after 2 doses of a Coronavirus Disease 2019 Vaccine: Community-Based, matched Cohort Study. Open. Forum Infect. Dis. 9 (9), ofac464. https://doi.org/10.1093/ofid/ofac464 (2022).
Al-Aly, Z., Bowe, B. & Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 28 (7), 1461–1467. https://doi.org/10.1038/s41591-022-01840-0 (2022).
Gao, P., Liu, J. & Liu, M. Effect of COVID-19 vaccines on reducing the risk of long COVID in the Real World: a systematic review and Meta-analysis. Int. J. Environ. Res. Public. Health. 19 (19), 12422. https://doi.org/10.3390/ijerph191912422 (2022).
Choi, B. C. & Pak, A. W. A catalog of biases in questionnaires. Prev. Chronic Dis. 2 (1), A13 (2005). (Epub 2004 Dec 15. PMID: 15670466; PMCID: PMC1323316).
Acknowledgements
We are grateful to the professionals in the Internal Medicine Service and the Infectious Diseases Unit at the University General Hospital of Castellón for their invaluable cooperation during the performance of the study, without which it would not have been possible. We also express our thanks to Meggan Harris for her assistance in editing.
Funding
No funding was obtained for the development of the study.
Author information
Authors and Affiliations
Contributions
-IPC: conception and design of the study, writing of the manuscript, bibliographic search, data collection and analysis and interpretation of data.-CRM: conception and design of the study, writing of the manuscript, bibliographic search, data collection and analysis and interpretation of data.-SFE: data collection and bibliographic search.-ASF: data collection and bibliographic search.-MVV: data collection and bibliographic search.-SFJ: data collection and bibliographic search.-EDB: data collection and bibliographic search. -GHR: data collection and bibliographic search.-MJEG: data collection and bibliographic search.-DPS: data collection and bibliographic search.-ACA: data collection and bibliographic search.-MLMC: conception and design of the study, writing of the manuscript.-JUB: conception and design of the study, writing of the manuscript.-JMRR: conception and design of the study, writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Pérez Catalán, I., Roig Martí, C., Folgado Escudero, S. et al. Presence of COVID-19 self-reported symptoms at 12 months in patients discharged from hospital in 2020–2021: a Spanish cross-sectional study. Sci Rep 14, 26575 (2024). https://doi.org/10.1038/s41598-024-78017-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-78017-x