Abstract
Numerous studies have reported vascular endothelial growth factor A (VEGF-A) has a significant impact on the pathophysiology of COVID-19. The objective of this systematic review and meta-analysis is to determine the prognostic value of increased levels of VEGF-A in individuals with COVID-19. A systematic literature search was conducted across multiple electronic databases, including PubMed, Web of Science, Cochrane Library, Scopus, EMBASE, and Google Scholar, up to January 2024. Studies examining the levels of VEGF-A in the serum or plasma of COVID-19 patients were incorporated, with specific attention given to contrasting severe/critical cases against moderate cases. Standardized mean differences (SMD) with 95% confidence intervals (CIs) were calculated using a random-effects model to determine overall effect sizes. Meta-regressions and subgroup analyses were performed to explore potential sources of heterogeneity. The meta-analysis synthesized data from 11 studies involving a total of 1119 COVID-19 patients. Elevated levels of VEGF-A were significantly associated with disease severity, with a pooled SMD of 0.525 (95% CI 0.239–0.058; P = 0.028). Research has indicated that the nature of the relationship differs among various age groups, and there were minor discrepancies in the techniques employed to obtain VEGF-A measurements. Furthermore, meta-regression analysis indicated a potential correlation between VEGF-A levels and assay technique and body mass index (BMI). This meta-analysis provides compelling evidence for the prognostic potency of VEGF-A in COVID-19. Understanding the intricate interplay between VEGF-A and COVID-19 pathophysiology holds promise for the development of targeted therapeutic strategies and prognostic indicators in the management of COVID-19.
Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Avoid common mistakes on your manuscript.
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a potent pathogen within the Coronaviridae family and is recognized as the primary etiological factor responsible for the onset of coronavirus disease 2019 (COVID-19). The COVID-19 pandemic emerged in 2019 and rapidly escalated into a global health crisis, inflicting widespread devastation on communities and claiming millions of lives [1, 2]. This virus infects type II alveolar epithelial cells in the lungs [3, 4]. Although COVID-19 primarily affects the respiratory tract, abundant evidence shows that COVID-19 affects multiple organs and causes a wide range of clinical symptoms [3]. Within the realm of COVID-19, considerable focus has been directed toward the complex relationship involving vascular function and the severity of the condition. The severity of COVID-19 has been associated with vascular complications such as cardiac and renal failure, coagulopathy, pulmonary embolism, and the onset of acute respiratory distress syndrome (ARDS) [4, 5]. The development of ARDS is associated with substantial damage to both endothelial and epithelial cells. Consequently, the interaction between the virus and the endothelium could potentially lead to a systemic syndrome caused by COVID-19, characterized by endothelial and vascular dysfunction.
According to recent studies, COVID-19 patients experience interwoven angiogenesis in their lungs, which confirms the presence of endothelial damage [6, 7]. The concentration of vascular endothelial growth factor (VEGF) in the serum has emerged as a significant and potential biomarker choice. VEGF, which is recognized as an angiocrine factor, has attracted interest as a prognostic marker in the context of COVID-19 [8, 9]. Despite the observed correlation between serum VEGF levels and the severity of COVID-19, the exact mechanistic implications of VEGF on the pathophysiology of the disease remain unclear [10, 11]. Given these intriguing observations, this systematic review and meta-analysis attempts to determine the prognostic value of elevated VEGF in COVID-19 by summarizing existing evidence and analyzing the clinical relevance of serum VEGF levels in COVID-19 patients.
Methods
Registration and protocol
This review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (updated in 2021) without significant deviations from the standard protocol. Furthermore, the systematic review has been duly registered in PROSPERO, an internationally recognized database for prospectively registered systematic reviews [CRD42023469722] [12, 13].
Literature search & eligibility criteria
The search strategy was refined to encompass a broader scope by incorporating various synonyms and related terms. The following keywords and phrases were utilized: (“vascular endothelial growth factor” OR “VEGF” OR “VEGF-A”) AND (“COVID-19” OR “SARS-CoV-2” OR “coronavirus disease 2019” OR “novel coronavirus” OR “pandemic respiratory infection caused by SARS-CoV-2” OR “COVID-19 virus” OR “severe acute respiratory syndrome coronavirus 2” OR “2019-nCoV”). The search was conducted until January 2024 and included databases such as PubMed, Web of Science, Cochrane Central, EMBASE, and Scopus. A detailed explanation of the database search strategy is available in Supplemental Table 1. The inclusion criteria for studies comprised the following: (I) observational research studies that examined endothelial dysfunction biomarkers in COVID-19 patients aged 18 years and above, (II) studies primarily employing reverse transcriptase polymerase chain reaction for COVID-19 detection, (III) studies published in the English language, (IV) studies that reported soluble forms of biomarkers, (V) studies that compared clinical outcomes between COVID-19 patients with adverse and favorable outcomes, and (VI) studies that provided baseline values of the investigated biomarkers within the initial 72 h of hospital admission. Conversely, studies were excluded if they met any of the following criteria: (I) reviews, editorials, case reports, comments, guidelines, systematic reviews, and meta-analyses, (II) pre-print and unpublished studies, (III) studies lacking pertinent data, or (IV) studies that compared COVID-19 patients with individuals not affected by COVID-19. In order to enhance the comprehensiveness of the analysis, the reference lists of selected studies were scrutinized for additional relevant publications.
Study selection
Two independent reviewers, S.B. and M.A., evaluated the eligibility of the studies using a systematic approach. Initially, they screened the titles and abstracts against predefined inclusion and exclusion criteria, which were developed in accordance with the study extraction framework outlined in our team’s prior research [14,15,16,17,18]. Following this preliminary screening, the reviewers conducted a detailed assessment of the full-text articles. Any disagreements between the two reviewers were resolved through discussion, and when necessary, by consulting a third reviewer to ensure consistency and accuracy.
Data extraction
A comprehensive data extraction was carried out to collect all necessary information, which includes the names of authors, publication year, the number of patients, patient characteristics, study design, and baseline levels of the VEGF-A biomarker that was being investigated. The patients were categorized into two groups based on the severity of their disease and clinical outcomes. The first group consisted of patients with unfavorable outcomes, including those who were labeled as “severe,” “intensive care,” “critical,” “non-survivors,” “ICU patients,” “patients on mechanical ventilation (MV),” and “patients with thromboembolic events.” The second group comprised patients with favorable outcomes, including those described as “non-severe,” “non-ICU patients,” “inpatients,” “moderate,” “mild,” “noncritical,” “survivors,” “patients without MV,” and “patients without thromboembolic events” (Table 1).
Quality assessment
For the quality assessment of the studies included in our meta-analysis, we utilized the Newcastle–Ottawa Scale (NOS) for non-randomized studies. This scale is designed to assess the quality of observational studies with three broad criteria: selection of the study groups, comparability of the groups, and the ascertainment of either the exposure or outcome of interest (Supplemental Table 2).
Each study was awarded a star for each quality item appropriately addressed, with a maximum of nine stars. The quality of each study was categorized based on the number of stars: Studies with 0–3 stars were considered of low quality, 4–6 stars of moderate quality, and 7–9 stars of high quality. Studies of low quality were subjected to sensitivity analyses to determine the impact of their inclusion on the overall meta-analytical findings. Additional measures included a graphical exploration of funnel plots to assess publication bias and conducting sensitivity analyses to explore the influence of individual studies on the overall meta-analysis results. This robust approach ensured that our conclusions were based on data of the highest integrity and relevance to clinical and public health recommendations [31]. Furthermore, Table 1 represents the Grading of Recommendations Development, Assessment, and Evaluation technique (GRADE), which were employed to appraise the status and grade of the studies incorporated in this meta-analysis.
Statistical analysis
The meta-analysis utilized Comprehensive Meta-Analysis (CMA) Version 4 software to facilitate statistical analyses. We employed standardized mean differences (SMD) ± standard deviation (SD) derived from baseline values of VEGF-A biomarkers to calculate pooled SMD and their corresponding 95% confidence intervals between COVID-19 patients with adverse and favorable outcomes [32, 33]. The heterogeneity among the studies was evaluated using the Chi-square Q test and the I-squared statistic. The I-squared statistic was then categorized as low (less than 25%), moderate (between 25 and 50%), or high (greater than 75%). We also planned to use a hierarchical summary receiver operating characteristic (H-SROC) analysis to evaluate the biomarkers’ sensitivity and specificity in predicting poor outcomes in COVID-19 patients. We also summarized the reported data for the area under the receiver operating curve, as well as the sensitivity and specificity of the studies included in our analysis. Subgroup analyses and meta-regressions were conducted to identify potential sources of variation in the data. Furthermore, the presence of publication bias was evaluated using the Egger and Begg tests, with a significance threshold of P < 0.05. The trim-and-fill method was used to estimate potentially missing studies due to publication bias in the funnel plot and to adjust the overall effect estimate.
Results
Selection process and characteristics of the included studies
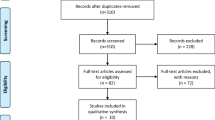
The systematic search identified 1671 articles across various databases. After removing 406 duplicates, 1265 articles were reviewed by title and abstract. This screening led to the exclusion of 1194 articles that did not meet the eligibility criteria. The remaining 71 articles underwent full-text review, resulting in the exclusion of 60 for reasons such as lack of relevant outcomes related to VEGF-A levels, absence of a control group, or insufficient data on COVID-19 prognosis. Consequently, 11 studies were included in the meta-analysis, encompassing a total of 1119 participants (diagnosed with severe COVID-19 and 587 with mild to moderate COVID-19). The flow of study selection is illustrated in Fig. 1.
Quality assessment
The quality scores of the studies included in the meta-analysis are presented in Supplemental Table 2. The NOS was utilized to evaluate the methodological quality of the included studies, with a maximum attainable score of 9. The included studies demonstrated variable quality, with scores ranging from 5 to 8. Specifically, studies such as Tsuji et al. [22] and Vassiliou et al. [30] received high-quality scores of 8, indicating robust methodology and comprehensive reporting. In contrast, Mescht et al. [21] scored 5, reflecting certain methodological limitations, including selection of controls and ascertainment of exposure.
Key domains of strength included adequate definition of cases and ascertainment of exposure, where most studies scored consistently high. However, variability was observed in the representativeness of cases and the same response rate across studies, potentially influencing the meta-analysis outcomes. These findings emphasize the need for consistent methodology in future research on VEGF-A and COVID-19 prognosis.
Relationship between circulating VEGF-A levels and severity of COVID-19
Meta-analysis
Our systematic review and meta-analysis assessed the prognostic potency of serum VEGF-A levels in COVID-19 patients, integrating data from 11 studies involving a total sample size of 1119 participants. The main meta-analysis revealed a statistically significant elevation in serum VEGF-A levels among patients with poor COVID-19 outcomes compared to those with favorable outcomes. The SMD was 0.525, with a 95% confidence interval (CI) ranging from − 1.22 to 2.27, and a P value of 0.028. A substantial degree of heterogeneity was observed among the included studies (I2 = 90.44%, P < 0.001), indicative of variability in study populations, methodologies, or both, as illustrated in Fig. 2.
Prediction interval
The estimated effect size followed a normal distribution, resulting in a prediction interval spanning from − 1.23 to 2.28. This prediction interval is designed to encompass the effect size within 95% of analogous populations while accounting for variables such as sample size and research methodology. It is imperative to exercise caution when interpreting findings within this prediction interval, as the true effect size may vary within this range. Figure 3 provides a visual representation of the prediction interval of their efficacy (Figs. 4, 5, and 6).
Sensitivity analysis & publication bias
A meticulous sensitivity analysis was conducted to assess the robustness of the current study by systematically excluding individual studies. Through this approach, the analysis revealed a range of SMD values, spanning from 0.239 to 0.058, concerning the sensitivity analysis of VEGF-A levels in COVID-19 patients compared to control groups. The lower limit of the 95% confidence interval was calculated at − 0.055 to 0.115, while the upper limit extended from 0.731 to 1.089. Importantly, these values maintained the clarity of I2 statistics. The sensitivity analysis reaffirmed the validity of the overall treatment effect (Fig. 7). The sensitivity analysis showed that the findings remained stable without any significant changes, which provided confidence in their robustness. Furthermore, the assessments conducted for publication bias using Egger’s test, along with the evaluation of funnel plot bias, as shown in Fig. 8, indicated a lack of substantial evidence for publication bias. Both the Egger test (P = 0.1423) and the Begg test (P = 0.1109) showed no publication bias. Furthermore, Supplemental Table 3 provides detailed results from the trim-and-fill method, which offers additional insights into bias evaluation.
Subgroup analysis
Subgroup analysis was meticulously conducted to dissect the nuances in the relationship between serum VEGF-A levels and COVID-19 prognosis across various demographics and methodological approaches. The results, summarized in Fig. 4, revealed a differential impact of VEGF-A based on age, assay method, and sample type.
Age-based analysis: The analysis highlighted age as a significant modulator of VEGF-A levels. Patients 50 years and older exhibited a slight, though non-significant, increase in VEGF-A levels (SMD = 0.389; 95% CI − 0.090 to 0.868; P = 0.111). Conversely, younger individuals, specifically those below 25 years, showed a marginally higher elevation in VEGF-A levels compared to the control group, although this difference also did not reach statistical significance (SMD = 0.808; 95% CI − 0.488 to 2.104; P = 0.222).
Assay method analysis: The type of assay used for measuring VEGF-A levels significantly influenced the results. Subgroup analysis showed that patients analyzed using multiplex immunoassay techniques had notably higher levels of VEGF-A compared to those assessed with ELISA (SMD = 1.169; 95% CI 0.105–2.234; P = 0.031 for multiplex; SMD = 0.286; 95% CI − 0.039 to 0.612; P = 0.085 for ELISA).
Sample Type Analysis: The analysis differentiated between serum and plasma samples. Patients with serum samples showed no significant changes in VEGF-A levels (SMD = 0.744; 95% CI − 0.028 to 1.517; P = 0.059), whereas those with plasma samples exhibited a slight but not significant increase (SMD = 0.344; 95% CI − 0.116 to 0.804; P = 0.143).
Meta-regression analysis
Meta-regression was employed to further explore the determinants influencing VEGF-A levels among COVID-19 patients, focusing on publication year, region, total sample size, test method, BMI, study design, and NOS score. This crucial information is comprehensively presented in Table 2.
Publication Year, Region, and Total Sample Size: These factors displayed a positive correlation with VEGF-A levels, though none were statistically significant, indicating a trend toward higher VEGF-A levels in more recent studies, larger studies, and certain geographical areas.
Assay technique and BMI: Both factors showed a significant positive correlation with VEGF-A levels. Higher BMI was associated with increased VEGF-A levels (meta-regression coefficient: 0.972; 95% CI − 0.071 to 0.267; P = 0.025), possibly due to the inflammatory state induced by higher body fat content, which could exacerbate the immune response to COVID-19. Similarly, more sensitive test methods correlated with higher detected levels of VEGF-A (meta-regression coefficient: 0.924; 95% CI 0.135–1.710; P = 0.021), underlining the role of assay sensitivity in capturing disease severity markers.
Study design and NOS score: Study design and Newcastle–Ottawa Scale (NOS) scores demonstrated a negative correlation with VEGF-A levels, suggesting that higher-quality studies or specific designs tend to report lower VEGF-A levels (study design regression coefficient = − 0.805; 95% CI − 1.882 to 0.272; P = 0.143; NOS score regression coefficient = − 0.033; 95% CI − 0.537 to 0.471; P = 0.008). This could be due to more stringent controls and sampling protocols in higher-quality studies, reducing potential biases and variability in VEGF-A measurement.
Discussions
Meta-analysis overview
This comprehensive meta-analysis investigates the prognostic potency of VEGF-A in patients with COVID-19, involving a diverse sample of 1119 participants across multiple studies. The findings consistently indicate a significant elevation in serum VEGF-A levels in patients with severe COVID-19 outcomes compared to those with milder symptoms. The implications of these results are profound, suggesting that VEGF-A could serve as a potential biomarker for COVID-19 prognosis and severity. This discussion elucidates the underlying biological mechanisms, interprets the primary findings, and explores their implications for clinical practice and future research.
Interpretation of main findings
Our meta-analysis highlights a significant correlation between higher serum VEGF-A levels and poor outcomes in COVID-19 patients, with a standardized mean difference of 0.525. This suggests that VEGF-A may play a role in the pathogenesis or progression of severe COVID-19. The substantial heterogeneity observed (I2 = 90.44%) could be indicative of variability in the disease's impact based on demographic and clinical factors. Moreover, the prediction interval (− 1.23 to 2.28) underscores the variability in effect size, suggesting that while VEGF-A is generally predictive of poor outcomes, its specific utility might vary widely across different populations and clinical settings.
Deciphering study outcome patterns: meta-regression insights
Meta-regression analysis explored various factors influencing VEGF-A levels, including publication year, region, and total sample size. While these factors showed a positive correlation, they were not statistically significant, indicating potential trends over time and geographical variations in VEGF-A expression possibly due to evolving laboratory techniques or regional differences in COVID-19 strain virulence [34, 35]. Notably, a significant positive correlation was found with BMI and test method. Higher BMI, associated with an inflammatory state, showed increased VEGF-A levels. This suggests that obesity-related inflammation might amplify the severity of COVID-19 via enhanced VEGF-A pathways, exacerbating the immune response [27, 36]. Additionally, more sensitive test methods were correlated with higher detected levels of VEGF-A, emphasizing the critical role of assay sensitivity in capturing disease severity markers effectively. Conversely, study design and NOS score displayed a negative correlation with VEGF-A levels. This finding suggests that higher-quality studies or specific designs, which likely incorporate more stringent controls and sampling protocols, tend to report lower VEGF-A levels. This could reduce potential biases and variability in VEGF-A measurement, leading to more accurate assessments of its role in COVID-19 prognosis [37, 38]. These findings are consistent with prior research on other biomarkers, reinforcing the broader applicability of such associations in disease prognosis and highlighting the necessity of rigorous methods and study quality for reliable biomarker analysis in disease prognosis [39,40,41].
Prior research and contextualization
The current meta-analysis builds on a foundation of existing literature exploring the role of VEGF-A as a biomarker in various diseases, particularly in its well-established role in promoting angiogenesis and vascular permeability. Prior studies have also linked elevated VEGF-A levels with worse outcomes in respiratory diseases, such as ARDS, which shares some pathophysiological features with severe COVID-19, including hypoxia-induced vascular leakage and inflammation [42, 43]. Historically, research on VEGF-A in the context of infectious diseases has suggested that VEGF-A plays a significant role in the vascular and inflammatory responses to infections. For instance, increased VEGF-A levels have been observed in patients with H1N1 influenza and SARS, correlating with disease severity and outcomes [44, 45]. These precedents support the hypothesis that VEGF-A could be a critical mediator in the progression of COVID-19, particularly in its severe forms, where inflammatory and vascular complications dominate.
In the specific context of COVID-19, early studies have demonstrated that patients with severe outcomes exhibit significantly higher levels of VEGF-A compared to those with milder disease [23]. This correlation is thought to stem from role of VEGF-A in enhancing vascular permeability, thereby potentially exacerbating the pulmonary edema seen in severe COVID-19 cases [46]. VEGF-A interaction with immune modulation, specifically its effects on macrophage function and cytokine production, provides a plausible link to the cytokine storm syndrome frequently observed in severe COVID-19 patients [47].
Recent systematic reviews and meta-analyses prior to ours have provided mixed insights, with some studies indicating a strong prognostic value of elevated VEGF-A levels, while others pointed to a more nuanced role influenced by patient demographics, comorbidities, and disease severity. For example, a meta-analysis involving 7668 COVID-19 patients reported that while elevated VEGF-A levels were generally predictive of poor outcomes, significant heterogeneity across studies suggested that the prognostic utility of VEGF-A may depend on the population and local clinical practices [48]. This underscores the importance of context in interpreting VEGF-A levels, which our current analysis seeks to clarify further by including a more extensive and diverse dataset. These findings have profound implications, suggesting that VEGF-A holds promise as a potential target for therapeutic intervention. VEGF-A inhibitors, traditionally employed in oncology to impede tumor vasculature, could be repurposed to regulate the acute inflammatory response in COVID-19. Previous research has demonstrated their efficacy in mitigating the incidence or severity of complications such as ARDS and multiple organ failure [49,50,51]. However, given the pivotal role of VEGF-A in normal vascular function and wound healing, any therapeutic manipulation of its activity warrants cautious consideration.
Biological mechanisms underlying roles of VEGF-A in COVID-19 severity
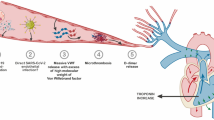
The underlying mechanisms connecting VEGF levels to the severity of COVID-19 involve both the factors that contribute to the elevation of VEGF in patients with COVID-19 and the subsequent impacts of heightened VEGF levels on the progression of the disease (Fig. 9).
VEGF-A is known to be upregulated in response to hypoxia, inflammation, and endothelial dysfunction, which are prominent features of severe COVID-19. Hypoxia, which results from impaired gas exchange in the lung due to viral-induced pneumonia, triggers the activation of hypoxia-inducible factor 1-alpha (HIF-1α) [52]. This transcription factor, in turn, stimulates the expression of VEGF-A to promote angiogenesis and improve tissue oxygenation [53]. In addition, the cytokine storms observed in severe COVID-19, characterized by elevated levels of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), directly stimulate VEGF-A secretion from activated endothelial and immune cells [54, 55]. Furthermore, the direct impact of the virus on endothelial cells in blood vessels can also increase VEGF-A expression. Endothelial dysfunction and injury trigger a compensatory response involving VEGF-A to promote angiogenesis and repair processes [56, 57].
On the other hand, the consequences of elevated VEGF-A levels in COVID-19 patients are multifaceted and can significantly influence disease severity and outcomes. In this context, one of the key mechanisms is promoting vascular permeability. Elevated VEGF-A levels disrupt endothelial cell junctions and increase fluid leakage into surrounding tissues [58, 59]. In patients with COVID-19, this vascular leakage leads to the development of pulmonary edema and ARDS, which are critical factors in disease progression toward severe respiratory failure [60]. Moreover, VEGF-A may exacerbate the hyperinflammatory response in COVID-19 patients by promoting immune cell recruitment and stimulating pro-inflammatory cytokine production [61,62,63]. This sustained inflammatory milieu contributes to the cytokine storm and multi-organ dysfunction observed in severe COVID-19 cases. Concurrently, VEGF-A can modulate adaptive immune responses in ways that impact viral clearance and disease resolution. Studies have shown elevated VEGF-A signaling is associated with T-cell exhaustion and dysfunction [64, 65]. This dysregulated immune response can worsen disease severity and increase susceptibility to secondary infections. VEGF-A is also implicated in regulating coagulation pathways. It is established that high VEGF-A levels can result in a hypercoagulable state by increasing the expression of tissue factor and the release of von Willebrand factor in endothelial cells [66, 67]. This effect can contribute to microvascular thrombosis and systemic coagulopathy, hallmarks of severe COVID-19. In addition, VEGF-A is a potent inducer of angiogenesis, the formation of new blood vessels. While this process is critical for tissue repair, excessive VEGF-A signaling can lead to aberrant angiogenesis and abnormal vascular remodeling. This may contribute to fibrotic changes in the lungs, potentially affecting long-term respiratory function in survivors [68]. In summary, elevated VEGF-A levels in COVID-19 patients reflect a complex interplay between viral infection, inflammation, hypoxia, and vascular dysfunction. This elevation contributes to the pathogenesis of severe disease manifestations, including ARDS, thrombosis, and immune dysregulation.
Clinical implications and future directions
The significant correlation between elevated VEGF-A levels and worse COVID-19 outcomes suggests that VEGF-A could serve as a valuable prognostic biomarker for identifying patients at risk of developing severe disease. This could facilitate earlier, more targeted therapeutic interventions, potentially improving patient outcomes. Furthermore, understanding the mechanistic role of VEGF-A in COVID-19 could open new avenues for therapeutic strategies aimed at modulating this growth factor to mitigate disease severity.
Future research should focus on longitudinal studies to track VEGF-A levels over the course of COVID-19, from initial infection through recovery or progression to severe disease. Such studies could clarify the temporal dynamics of VEGF-A expression and its prognostic utility. Additionally, interventional studies examining the effects of therapies targeting VEGF-A pathways could provide insights into their potential benefits or risks in managing COVID-19.
Strengths and limitations
This study boasts several strengths, including a meticulous search methodology, a substantial sample size drawn from multiple studies, and consistent findings that underscore the prognostic significance of VEGF-A within the COVID-19 landscape. The utilization of subgroup analyses and meta-regression techniques has enhanced the interpretation of results, thereby augmenting the relevance and generalizability of our findings.
However, several limitations warrant acknowledgment. Foremost among these is the variability in detection methods employed across the included studies, which may introduce discrepancies in reported VEGF-A levels. Despite efforts to standardize our analysis, variations in assay sensitivity, specificity, and calibration standards could potentially influence result consistency. Additionally, the evolving landscape of COVID-19 treatment modalities during the pandemic imposes further constraints on our analysis. Moreover, the influence of genetic factors, ethnicity, and geographical location on disease severity emerges as a significant area for future investigation. Furthermore, inconsistent reporting of therapeutic interventions, such as anticoagulants, steroids, and antiplatelets, is a significant limitation in our understanding of the impact of endothelial and angiogenesis biomarkers on COVID-19 prognosis. The potential effect of these medications on the observed associations between VEGF-A and COVID-19 prognosis is unclear, and further research is needed in this area.
Conclusions
In conclusion, this systematic review and meta-analysis provides valuable insights into the prognostic role of elevated VEGF-A in COVID-19. While the precise mechanisms require further investigation, our findings of the present investigation indicate that VEGF-A levels could represent a valuable biomarker to determine COVID-19 patients at risk of poor outcomes [SMD: 0.525; P = 0.028]. This information could assist with early management and risk stratification, eventually improving patient care and outcomes in the midst of the current COVID-19 epidemic.
Data availability
No datasets were generated or analyzed during the current study.
References
Carcaterra M, Caruso C. Alveolar epithelial cell type II as main target of SARS-CoV-2 virus and COVID-19 development via NF-Kb pathway deregulation: a physio-pathological theory. Med Hypotheses. 2021;146:110412.
Huang J, et al. SARS-CoV-2 infection of pluripotent stem cell-derived human lung alveolar type 2 cells elicits a rapid epithelial-intrinsic inflammatory response. Cell Stem Cell. 2020;27(6):962-973. e7.
Ding Z, et al. Unveiling the intricacies of COVID-19: Autoimmunity, multi-organ manifestations and the role of autoantibodies. Scand J Immunol. 2024;99(2):e13344.
Rodríguez C, et al. Pulmonary endothelial dysfunction and thrombotic complications in patients with COVID-19. Am J Respir Cell Mol Biol. 2021;64(4):407–15.
Mangalmurti NS, et al. COVID-19–associated acute respiratory distress syndrome clarified: A vascular endotype? Am J Respir Crit Care Med. 2020;202(5):750–3.
Mentzer SJ, Ackermann M, Jonigk D. Endothelialitis, microischemia, and intussusceptive angiogenesis in COVID-19. Cold Spring Harbor Perspect Med. 2022;12:a041157.
Ackermann M, et al. Inflammation and intussusceptive angiogenesis in COVID-19: everything in and out of flow. Eur Respir Soc. 2020;56:2003147.
Kong Y, et al. VEGF-D: a novel biomarker for detection of COVID-19 progression. Crit Care. 2020;24:1–4.
Yin X-X, et al. Vascular endothelial growth factor (VEGF) as a vital target for brain inflammation during the COVID-19 outbreak. ACS Chem Neurosci. 2020;11(12):1704–5.
Smadja DM, et al. Placental growth factor level in plasma predicts COVID-19 severity and in-hospital mortality. J Thromb Haemost. 2021;19(7):1823–30.
Korobelnik J-F, et al. Guidance for anti-VEGF intravitreal injections during the COVID-19 pandemic. Graefes Arch Clin Exp Ophthalmol. 2020;258:1149–56.
Rethlefsen ML, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):1–19.
Schiavo JH. PROSPERO: an international register of systematic review protocols. Med Ref Serv Q. 2019;38(2):171–80.
Bahreiny SS, et al. A closer look at Galectin-3: its association with gestational diabetes mellitus revealed by systematic review and meta-analysis. J Diabetes Metab Disord. 2024;23(2):1621–33.
Fard RM, Rashno M, Bahreiny SS. Effects of melatonin supplementation on markers of inflammation and oxidative stress in patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr ESPEN. 2024;63:530–9.
Bahreiny SS, et al. Exploring the relationship between Hashimoto’s thyroiditis and male fertility: a meta-analytic and meta-regression perspective on hormonal and seminal factors. Asian Pac J Reprod. 2024;13(4):147–59.
Bahreiny SS, et al. Meta-analytical and meta-regression evaluation of subclinical hyperthyroidism’s effect on male reproductive health: hormonal and seminal perspectives. Reprod Sci. 2024;31(10):2957–71.
Bahreiny SS, Ahangarpour A, Aghaei M. Circulating levels of advanced glycation end products in females with polycystic ovary syndrome: a meta-analysis. Reprod Dev Med. 2024;8(2):93–100.
Elseidy SA, et al. Cardiovascular complications in the post-acute COVID-19 syndrome (PACS). IJC Heart Vasc. 2022;40:101012.
Alfadda AA, et al. Early cytokine signatures of hospitalized mild and severe COVID-19 patients: a prospective observational study. J Inflamm Res. 2023;16:2631–43.
van der Mescht MA, et al. Comparison of platelet-and endothelial-associated biomarkers of disease activity in people hospitalized with Covid-19 with and without HIV co-infection. Front Immunol. 2023;14:1235914.
Tsuji M, et al. Serum VEGF levels on admission in COVID-19 patients correlate with SP-D and neutrophils, reflecting disease severity: a prospective study. medRxiv. 2023; 2023.07. 17.23292653.
Woolard J, et al. Molecular diversity of VEGF-A as a regulator of its biological activity. Microcirculation. 2009;16(7):572–92.
Tufa A, et al. Inflammatory mediators profile in patients hospitalized with COVID-19: a comparative study. Front Immunol. 2022;13:964179.
Rodrigues JK, et al. Marcadores séricos de estresse oxidativo e resultados dos procedimentos de reprodução assistida em pacientes inférteis com síndrome dos ovários policísticos e controles. Rev Bras Ginecol Obstet. 2010;32:118–25.
Moti M, Amini L, Ardakani SS, Kamalzadeh S, Masoomikarimi M. Oxidative stress and anti-oxidant defense system in Iranian women with polycystic ovary syndrome. Iran J Reprod Med. 2015;13(6):373.
Pine AB, et al. Circulating markers of angiogenesis and endotheliopathy in COVID-19. Pulm Circ. 2020;10(4):2045894020966547.
Smadja DM, et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis. 2020;23(4):611–20.
White D, et al. Evaluation of COVID-19 coagulopathy; laboratory characterization using thrombin generation and nonconventional haemostasis assays. Int J Lab Hematol. 2021;43(1):123–30.
Vassiliou AG, et al. ICU admission levels of endothelial biomarkers as predictors of mortality in critically ill COVID-19 patients. Cells. 2021;10(1):186.
Peterson, J., et al., The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa: Ottawa Hospital Research Institute, 2011. 2(1): p. 1–12.
Tinahones FJ, et al. Obesity-associated insulin resistance is correlated to adipose tissue vascular endothelial growth factors and metalloproteinase levels. BMC Physiol. 2012;12(1):1–8.
Mahdizade AH, et al. The influence of CDKAL1 (rs7754840) gene polymorphism on susceptibility to gestational diabetes mellitus in pregnant women: a systematic review and meta-analysis. Int J Diabetes Dev Ctries. 2024;44(1):3–12.
Polyakova M, et al. Racial disparities in excess all-cause mortality during the early COVID-19 pandemic varied substantially across states: study examines the geographic variation in excess all-cause mortality by race to better understand the impact of the COVID-19 pandemic. Health Aff. 2021;40(2):307–16.
Josuttis D, et al. Vascular endothelial growth factor as potential biomarker for COVID-19 severity. J Intensive Care Med. 2023;38:1165–73.
Loebig M, et al. Evidence for a relationship between VEGF and BMI independent of insulin sensitivity by glucose clamp procedure in a homogenous group healthy young men. PLoS ONE. 2010;5(9):e12610.
Jung RG, et al. Methodological quality of COVID-19 clinical research. Nat Commun. 2021;12(1):943.
Aghaei M, et al. The need to establish and recognize the field of clinical laboratory science (CLS) as an essential field in advancing clinical goals. Health Sci Rep. 2024;7(8):e70008.
Bahreiny SS, et al. Association of KCNJ11 (rs5219) gene polymorphism with susceptibility to gestational diabetes mellitus: a review and meta-analysis. Iran J Obstet Gynecol Infertil. 2024;27(1):63–79.
Bahreiny SS, et al. Prevalence of autoimmune thyroiditis in women with polycystic ovary syndrome: a systematic review and meta-analysis. Iran J Obstet Gynecol Infertil. 2023;26(1):94–106.
Bahreiny SS, et al. Autoimmune thyroid disorders and polycystic ovary syndrome: tracing links through systematic review and meta-analysis. J Reprod Immunol. 2024;163:104215.
Barratt S, Medford A, Millar A. Vascular endothelial growth factor in acute lung injury and acute respiratory distress syndrome. Respiration. 2014;87(4):329–42.
Abadie Y, et al. Decreased VEGF concentration in lung tissue and vascular injury during ARDS. Eur Respir J. 2005;25(1):139–46.
Bautista E, et al. Angiogenic and inflammatory markers in acute respiratory distress syndrome and renal injury associated to A/H1N1 virus infection. Exp Mol Pathol. 2013;94(3):486–92.
Madureira G, Soares R. The misunderstood link between SARS-CoV-2 and angiogenesis. A narrative review. Pulmonology. 2023;29(4):323–31.
Yazihan N, et al. Comparison of VEGF-A values between pregnant women with COVID-19 and healthy pregnancies and its association with composite adverse outcomes. J Med Virol. 2021;93(4):2204–9.
Tang Y, et al. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol. 2020;11:1708.
Mohebbi A, et al. Biomarkers of endothelial dysfunction are associated with poor outcome in COVID-19 patients: a systematic review and meta-analysis. Rev Med Virol. 2023;33(4):e2442.
Lampropoulou DI, et al. The potential role of the combined PARP-1 and VEGF inhibition in severe SARS-CoV-2 (COVID-19) infection. J Infect Dev Ctries. 2022;16(01):101–11.
Talotta R. Impaired VEGF-A-mediated neurovascular crosstalk induced by SARS-CoV-2 spike protein: A potential hypothesis explaining long COVID-19 symptoms and COVID-19 vaccine side effects? Microorganisms. 2022;10(12):2452.
Sahebnasagh A, et al. Anti-VEGF agents: as appealing targets in the setting of COVID-19 treatment in critically ill patients. Int Immunopharmacol. 2021;101:108257.
Raitsev S, et al. Study of HIF-1α-signaling pathway components in plasma of patients with COVID-19 infection of different degrees of severity. Probl Biol Med Pharm Chem. 2024;27(4):57–62.
Zhang W, et al. Mechanism of the HIF-1α/VEGF/VEGFR-2 pathway in the proliferation and apoptosis of human haemangioma endothelial cells. Int J Exp Pathol. 2023;104(5):258–68.
Lu P, et al. Critical role of TNF-α-induced macrophage VEGF and iNOS production in the experimental corneal neovascularization. Invest Ophthalmol Vis Sci. 2012;53(7):3516–26.
Loeffler S, et al. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int J Cancer. 2005;115(2):202–13.
Xu S-W, Ilyas I, Weng J-P. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol Sin. 2023;44(4):695–709.
Bastani M-N, et al. Update prognostic potency of surfactant protein D (SP-D) in the COVID-19 landscape: an in-depth meta-analytical exploration. Biomark Med. 2024;18(24):1135–48.
Ashina K, et al. VEGF-induced blood flow increase causes vascular hyper-permeability in vivo. Biochem Biophys Res Commun. 2015;464(2):590–5.
Tomita K, et al. Vascular endothelial growth factor contributes to lung vascular hyperpermeability in sepsis-associated acute lung injury. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:2365–74.
Kristensen MK, et al. Cell adhesion molecules and vascular endothelial growth factor at the systemic and alveolar level in coronavirus disease 2019 acute respiratory distress syndrome. J Infect Dis. 2021;224(6):1101–3.
Murakami M, et al. VEGFR1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arterioscler Thromb Vasc Biol. 2008;28(4):658–64.
Marumo T, Schini-Kerth VB, Busse R. Vascular endothelial growth factor activates nuclear factor-kappaB and induces monocyte chemoattractant protein-1 in bovine retinal endothelial cells. Diabetes. 1999;48(5):1131–7.
Yoo S-A, et al. Arginine-rich anti-vascular endothelial growth factor (anti-VEGF) hexapeptide inhibits collagen-induced arthritis and VEGF-stimulated productions of TNF-α and IL-6 by human monocytes. J Immunol. 2005;174(9):5846–55.
Voron T, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212(2):139–48.
Kim CG, et al. VEGF-A drives TOX-dependent T cell exhaustion in anti-PD-1-resistant microsatellite stable colorectal cancers. Sci Immunol. 2019;4(41):eaay0555.
Mechtcheriakova D, et al. Specificity, diversity, and convergence in VEGF and TNF-α signaling events leading to tissue factor up regulation via EGR-1 in endothelial cells. FASEB J. 2001;15(1):230–42.
Xiong Y, et al. Vascular endothelial growth factor (VEGF) receptor-2 tyrosine 1175 signaling controls VEGF-induced von Willebrand factor release from endothelial cells via phospholipase C-γ1-and protein kinase A-dependent pathways. J Biol Chem. 2009;284(35):23217–24.
Philippe A, et al. VEGF-A plasma levels are associated with impaired DLCO and radiological sequelae in long COVID patients. Angiogenesis. 2024;27(1):51–66.
Acknowledgements
The authors would like to express their appreciation to the researchers whose articles were used in the present research.
Funding
The author(s) received no financial support for this research.
Author information
Authors and Affiliations
Contributions
In this study, MNB and SB performed data analysis and wrote the first draft of the manuscript. The study was formulated, designed, and overseen by MNS, MA; MA and RM also carried out data analysis, visualized results, and contributed to the manuscript’s critical revision and editing. SB, MNB, and HK supervised and reviewed the project. Finally, all authors reviewed and approved the final version of the manuscript before its submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bahreiny, S.S., Bastani, MN., Keyvani, H. et al. VEGF-A in COVID-19: a systematic review and meta-analytical approach to its prognostic value. Clin Exp Med 25, 81 (2025). https://doi.org/10.1007/s10238-025-01583-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10238-025-01583-5