Abstract
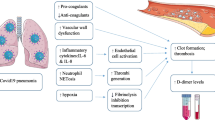
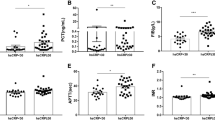
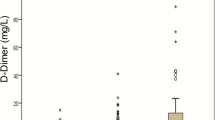
COVID-19, caused by SARS-CoV-2 infection, frequently induces thrombotic complications in affected individuals. P-selectin, a pivotal platelet marker, plays a central role in platelet–leukocyte aggregation, contributing to hemostasis and thrombosis. Additionally, D-dimer serves as an indicator of coagulation system activity, while miR-17-5p exhibits antiviral properties in respiratory infections. This study aimed to evaluate and compare the expression levels of D-dimer, P-selectin, and miR-17-5p in COVID-19 patients hospitalized in intensive care units (ICUs) and those in non-ICU wards. This cross-sectional study included 50 COVID-19 patients, divided into ICU and non-ICU groups. P-selectin expression was assessed using Flow cytometry, D-dimer levels were measured via chemiluminescence, and miR-17-5p expression was analyzed using quantitative real-time polymerase chain reaction (qRT-PCR). Our analysis revealed no significant difference in P-selectin expression levels between ICU and non-ICU patients (p = 0.1068). However, the expression levels of D-dimer and miR-17-5p were significantly elevated in ICU patients compared to non-ICU patients, with corresponding p-values of 0.032 and 0.0176, respectively. The heightened expression of D-dimer and miR-17-5p in ICU patients suggests their potential utility as predictive biomarkers for assessing the hemostatic status of COVID-19 patients.




Similar content being viewed by others
Explore related subjects
Discover the latest articles and news from researchers in related subjects, suggested using machine learning.Data Availability
No datasets were generated or analysed during the current study.
References
Agrati C, Bordoni V, Sacchi A, Petrosillo N, Nicastri E, Del Nonno F et al (2021) Elevated P-Selectin in severe Covid-19: considerations for therapeutic options. Mediterranean J Hematol Infectious Dis 13(1):e2021016
Alberio L, Dale GL (1998) Flow cytometric analysis of platelet activation by different collagen types present in the vessel wall. Br J Haematol 102(5):1212–1218
Andersen CL, Jensen JL, Ørntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Can Res 64(15):5245–5250
Calderon-Dominguez M, Trejo-Gutierrez E, González-Rovira A, Beltrán-Camacho L, Rojas-Torres M, Eslava-Alcón S et al (2022) Serum microRNAs targeting ACE2 and RAB14 genes distinguish asymptomatic from critical COVID-19 patients. Molecular Therapy-Nucleic Acids 29:76–87
Cavagna E, Muratore F, Ferrari F (2020) Pulmonary thromboembolism in COVID-19: venous thromboembolism or arterial thrombosis? Radiol: Cardiothoracic Imaging. 2(4):e200289
de Gonzalo-Calvo D, Benítez ID, Pinilla L, Carratalá A, Moncusí-Moix A, Gort-Paniello C et al (2021) Circulating microRNA profiles predict the severity of COVID-19 in hospitalized patients. Transl Res 236:147–159
Demiray A, Sarı T, Çalışkan A, Nar R, Aksoy L, Akbubak İH (2021) Serum microRNA signature is capable of predictive and prognostic factor for SARS-COV-2 virulence. Turkish J Biochem 46(3):245–253
Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS et al (2020) Coronavirus disease 2019–COVID-19. Clin Microbiol Rev. https://doi.org/10.1128/cmr.00028-20
Di Micco P, Russo V, Carannante N, Imparato M, Cardillo G, Lodigiani C (2020) Prognostic value of fibrinogen among COVID-19 patients admitted to an emergency department: an Italian cohort study. J Clin Med 9(12):4134
Eljilany I, Elzouki A-N (2020) D-dimer, fibrinogen, and IL-6 in COVID-19 patients with suspected venous thromboembolism: a narrative review. Vascular Health Risk Manag 16:455–462
Fayyad-Kazan M, Makki R, Skafi N, El Homsi M, Hamade A, El Majzoub R et al (2021) Circulating miRNAs: potential diagnostic role for coronavirus disease 2019 (COVID-19). Infect Genet Evol 94:105020
Fraser DD, Patterson EK, Slessarev M, Gill SE, Martin C, Daley M et al (2020) Endothelial injury and glycocalyx degradation in critically ill coronavirus disease 2019 patients: implications for microvascular platelet aggregation. Crit Care Exp 2(9):e0194
García-Hidalgo MC, González J, Benítez ID, Carmona P, Santisteve S, Pérez-Pons M et al (2022) Identification of circulating microRNA profiles associated with pulmonary function and radiologic features in survivors of SARS-CoV-2-induced ARDS. Emerging Microbes Infections 11(1):1537–1549
Goshua G, Pine AB, Meizlish ML, Chang C-H, Zhang H, Bahel P et al (2020) Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol 7(8):e575–e582
Hanff TC, Mohareb AM, Giri J, Cohen JB, Chirinos JA (2020) Thrombosis in COVID-19. Am J Hematol 95(12):1578–1589
Harenberg J, Favaloro E (2020) COVID-19: progression of disease and intravascular coagulation–present status and future perspectives. Clin Chem Laboratory Med (CCLM) 58(7):1029–1036
He F, Deng Y, Li W (2020) Coronavirus disease 2019: What we know? J Med Virol 92(7):719–725
Hemida MG, Ye X, Thair S, Yang D (2010) Exploiting the therapeutic potential of microRNAs in viral diseases: expectations and limitations. Mol Diagn Ther 14:271–282
Jankowska KI, Sauna ZE, Atreya CD (2020) Role of microRNAs in hemophilia and thrombosis in humans. Int J Mol Sci 21(10):3598
Jenne C, Urrutia R, Kubes P (2013) Platelets: bridging hemostasis, inflammation, and immunity. Int J Lab Hematol 35(3):254–261
Keikha R, Hashemi-Shahri SM, Jebali A (2021) The relative expression of miR-31, miR-29, miR-126, and miR-17 and their mRNA targets in the serum of COVID-19 patients with different grades during hospitalization. Eur J Med Res 26:1–8
Khan MAAK, Sany MRU, Islam MS, Islam ABMMK (2020) Epigenetic regulator miRNA pattern differences among SARS-CoV, SARS-CoV-2, and SARS-CoV-2 world-wide isolates delineated the mystery behind the epic pathogenicity and distinct clinical characteristics of pandemic COVID-19. Front Genetics 11:549561
Khatri P, Agrawal KK, Sharma D, Chhetri P, Neupane A, Piriyani RM et al (2021) Prevalence of elevated D-dimer levels in confirmed COVID-19 cases in intensive care unit of a tertiary care centre of western Nepal. J Nepal Med Assoc 59(235):243
Lippi G, Favaloro EJ (2013) Laboratory hemostasis: milestones in clinical chemistry and laboratory medicine. Clin Chem Laboratory Med (CCLM) 51(1):91–97
Lippi G, Salvagno G, Rossi L, Montagnana M, Franchini M, Guidi G (2007) Analytical performances of the D-dimer assay for the Immulite 2000 automated immunoassay analyser. Int J Lab Hematol 29(6):415–420
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25(4):402–408
Manne BK, Münzer P, Badolia R, Walker-Allgaier B, Campbell RA, Middleton E et al (2018) PDK1 governs thromboxane generation and thrombosis in platelets by regulating activation of Raf1 in the MAPK pathway. J Thromb Haemost 16(6):1211–1225
Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C et al (2020) Platelet gene expression and function in patients with COVID-19. Blood J Am Soc Hematol 136(11):1317–1329
Marchandot B, Sattler L, Jesel L, Matsushita K, Schini-Kerth V, Grunebaum L et al (2020) COVID-19 related coagulopathy: a distinct entity? J Clin Med 9(6):1651
Moc C (2020) Coagulopathy in COVID-19: manifestations and management. Clevel Clin J Med 87(8):461
Mukhopadhyay D, Mussa BM (2020) Identification of novel hypothalamic microRNAs as promising therapeutics for SARS-CoV-2 by regulating ACE2 and TMPRSS2 expression: an in silico analysis. Brain Sci 10(10):666
Paul S, Vázquez LAB, Reyes-Pérez PR, Estrada-Meza C, Alburquerque RAA, Pathak S et al (2022) The role of microRNAs in solving COVID-19 puzzle from infection to therapeutics: a mini-review. Virus Res 308:198631
Petito E, Falcinelli E, Paliani U, Cesari E, Vaudo G, Sebastiano M et al (2021) Association of neutrophil activation, more than platelet activation, with thrombotic complications in coronavirus disease 2019. J Infect Dis 223(6):933–944
Polgar J, Matuskova J, Wagner D (2005) The P-selectin, tissue factor, coagulation triad. J Thromb Haemost 3(8):1590–1596
Qin C, Wang J, Wei Q, She M, Marasco WA, Jiang H et al (2005) An animal model of SARS produced by infection of Macaca mulatta with SARS coronavirus. J Pathol 206(3):251–259
Sakr Y, Giovini M, Leone M, Pizzilli G, Kortgen A, Bauer M et al (2020) Pulmonary embolism in patients with coronavirus disease-2019 (COVID-19) pneumonia: a narrative review. Ann Intensive Care 10:1–13
Sardar R, Satish D, Gupta D (2020) Identification of novel SARS-CoV-2 drug targets by host microRNAs and transcription factors co-regulatory interaction network analysis. Front Genet 11:571274
Skalsky RL, Cullen BR (2010) Viruses, microRNAs, and host interactions. Annu Rev Microbiol 64:123–141
Spadaro S, Fogagnolo A, Campo G, Zucchetti O, Verri M, Ottaviani I et al (2021) Markers of endothelial and epithelial pulmonary injury in mechanically ventilated COVID-19 ICU patients. Crit Care 25:1–9
Weng Z, Li D, Zhang L, Chen J, Ruan C, Chen G et al (2010) PTEN regulates collagen-induced platelet activation. Blood J Am Soc Hematol 116(14):2579–2581
Xiong M, Liang X, Wei YD (2020) Changes in blood coagulation in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Br J Haematol 189(6):1050
Yun S-H, Sim E-H, Goh R-Y, Park J-I, Han J-Y (2016) Platelet activation: the mechanisms and potential biomarkers. BioMed Res Int. https://doi.org/10.1155/2016/9060143
Zhang H, Liu Y, Yu B, Lu R (2023) An optimized TRIzol-based method for isolating RNA from adipose tissue. Biotechniques 74(5):203–209
Acknowledgements
The authors wish to express their sincere gratitude to Shiraz University of Medical Sciences for their invaluable support in facilitating the execution of this study. Their assistance and resources have been instrumental in enabling our research endeavors and contributing to the advancement of medical science. We are deeply appreciative of their dedication to fostering academic excellence and their commitment to promoting research initiatives.
Funding
This study was supported by Shiraz University of Medical Sciences (Shiraz, Iran) (grant number: 23551).
Author information
Authors and Affiliations
Contributions
G.T. and M.M. contributed to the study concept and design. S.A. and P.T. were involved in methodology development. G.T. and V.S. contributed to software development. G.T. and P.T. performed validation procedures. V.S. conducted formal analysis. N.S. conducted the investigation. M.M. provided resources. G.T. and M.M. curated the data. G.T. and V.S. prepared the original draft of the manuscript. G.T. supervised the project and provided visualization and project administration. All authors gave final approval to the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethical Approval
Ethical approval for this experimental study was obtained from the Ethics Committee of Shiraz University of Medical Sciences under the code IR.SUMS.REC.1400.461.
Consent to Participate
All participants involved in the study provided informed consent prior to their involvement, as per ethical guidelines.
Consent to Publications
Informed consent was obtained from all individual participants for publication of any data from this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shirani Asl, V., Moghaddami, M., Abbasi, S. et al. Evaluation of D-Dimer, P-Selectin, and miR-17-5p Expression in ICU and Non-ICU COVID-19 Patients: A Cross-sectional Study. Biochem Genet (2025). https://doi.org/10.1007/s10528-025-11062-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10528-025-11062-x