Jeopardy of COVID-19: Rechecking the Perks of Phytotherapeutic Interventions
Abstract
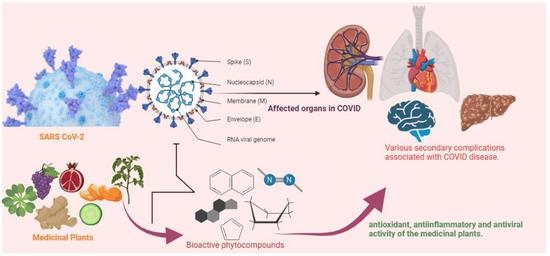
:1. Introduction
2. Insight into the Pathogenesis of COVID-19
3. Potential Targets of Medicinal Plants in COVID-19 Pathogenesis
3.1. Viral Attachment
3.2. Encapsulation, Replication of the Virus and Viral Protease
3.3. Inflammation
3.4. Oxidative Stress
3.5. Immune Response
4. Effect of Medicinal Plants in COVID-19 Associated Secondary Diseases
4.1. Acute Kidney Damage
4.2. Cardiovascular Disease
4.3. Respiratory Disease
4.4. Neurological Disease
4.5. Liver Disease
4.6. Others
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, B.; Biswas, A. Status evaluation of provinces affected by COVID-19: A qualitative assessment using fuzzy system. Appl. Soft Comput. 2021, 109, 107540. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Ali, K.; Connell, D.; Mordi, I.R.; George, J.; Lang, E.M.; Lang, C.C. COVID-19-Associated Cardiovascular Complications. Diseases 2021, 9, 47. [Google Scholar] [CrossRef]
- Zacharias, H.; Dubey, S.; Koduri, G.; D’Cruz, D. Rheumatological complications of Covid 19. Autoimmun. Rev. 2021, 20, 102883. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.-Y.; Wang, B.; Liu, B.-C. Acute kidney injury in the 2019 novel coronavirus disease. Kidney Dis. 2020, 6, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Darenskaya, M.; Kolesnikova, L.; Kolesnikov, S. The Association of Respiratory Viruses with Oxidative Stress and Antioxidants. Implications for the COVID-19 Pandemic. Curr. Pharm. Des. 2021, 27, 1618–1627. [Google Scholar] [CrossRef] [PubMed]
- Mehri, F.; Rahbar, A.H.; Ghane, E.T.; Souri, B.; Esfahani, M. The comparison of oxidative markers between Covid-19 patients and healthy subjects: Oxidative stress and Covid-19. Arch. Med. Res. 2021. [Google Scholar] [CrossRef]
- Esper, F.P.; Cheng, Y.-W.; Adhikari, T.M.; Tu, Z.J.; Li, D.; Li, E.A.; Farkas, D.H.; Procop, G.W.; Ko, J.S.; Chan, T.A. Genomic epidemiology of SARS-CoV-2 infection during the initial pandemic wave and association with disease severity. JAMA Netw. Open 2021, 4, e217746. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Banerjee, A.K.; Tripathi, P.P.; Srivastava, A.K.; Ray, U. A virus that has gone viral: Amino acid mutation in S protein of Indian isolate of Coronavirus COVID-19 might impact receptor binding, and thus, infectivity. Biosci. Rep. 2020, 40, BSR20201312. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; Lam, E.C.; Astudillo, M.G.; Yang, D.; Miller, T.E.; Feldman, J.; Hauser, B.M.; Caradonna, T.M.; Clayton, K.L.; Nitido, A.D. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 2021, 184, 476–488.e11. [Google Scholar] [CrossRef] [PubMed]
- Ntyonga-Pono, M.-P. COVID-19 infection and oxidative stress: An under-explored approach for prevention and treatment? Pan Afr. Med. J. 2020, 35 (Suppl. 2), 12. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [Green Version]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: Considerations during the COVID-19 crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K. Medicinal plants: Future source of new drugs. Int. J. Herb. Med. 2016, 4, 59–64. [Google Scholar]
- Sen, B. Potentiality and possibility of Medicinal Plants on Ayurvedic Principle in prevention and treatment of COVID-19. J. Ayu. Herb. Med. 2020, 6, 100–107. [Google Scholar] [CrossRef]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Smith, P.T.; Bartlett, J.; Shanmugam, K.; Münch, G.; Wu, M. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J. Agric. Food Chem. 2011, 59, 12361–12367. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Choudhury, P.R.; Das, S.; Talukdar, A.D.; Choudhury, M.D. In vitro antioxidant activity of bark extracts of Oroxylum indicum (L.) vent. Asian J. Pharm. Clin. Res. 2017, 10, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Atawodi, S. Antioxidant potential of African medicinal plants. Afr. J. Biotechnol. 2005, 4, 128–133. [Google Scholar]
- Murugesan, D.; Deviponnuswamy, R. Potential anti-inflammatory medicinal plants-a review. Int. J. Pharm. Pharm. Sci. 2014, 6, 43–49. [Google Scholar]
- Siriwatanametanon, N.; Fiebich, B.L.; Efferth, T.; Prieto, J.M.; Heinrich, M. Traditionally used Thai medicinal plants: In vitro anti-inflammatory, anticancer and antioxidant activities. J. Ethnopharmacol. 2010, 130, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Galloway, S.E.; Paul, P.; MacCannell, D.R.; Johansson, M.A.; Brooks, J.T.; MacNeil, A.; Slayton, R.B.; Tong, S.; Silk, B.J.; Armstrong, G.L.; et al. Emergence of SARS-CoV-2 b. 1.1. 7 lineage—United States, December 29, 2020–January 12, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.V.; van Goor, H. Ireland, Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Rahman, N.; Basharat, Z.; Yousuf, M.; Castaldo, G.; Rastrelli, L.; Khan, H. Virtual screening of natural products against type II transmembrane serine protease (TMPRSS2), the priming agent of coronavirus 2 (SARS-CoV-2). Molecules 2020, 25, 2271. [Google Scholar] [CrossRef] [PubMed]
- Karcioglu, O.; Afacan, G.; Ozkaya, B.; Yilmaz, E.; Ersan, E.; Yeniocak, S.; Hosseinzadeh, M. Cytokine storm, corticosteroids and interleukin-6 receptor antibodies in context of antiinflammatory treatment in COVID-19. J. Pharm. Res. Int. 2020, 32, 67–75. [Google Scholar] [CrossRef]
- Ma, H.; Zeng, W.; He, H.; Zhao, D.; Jiang, D.; Zhou, P.; Cheng, L.; Li, Y.; Ma, X.; Jin, T. Serum IgA, IgM, and IgG responses in COVID-19. Cell. Mol. Immunol. 2020, 17, 773–775. [Google Scholar] [CrossRef]
- Remy, K.E.; Mazer, M.; Striker, D.A.; Ellebedy, A.H.; Walton, A.H.; Unsinger, J.; Blood, T.M.; Mudd, P.A.; Yi, D.J.; Mannion, D.A. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight 2020, 5, e140329. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Vaninov, N. In the eye of the COVID-19 cytokine storm. Nat. Rev. Immunol. 2020, 20, 277. [Google Scholar] [CrossRef]
- Sun, Z.; He, G.; Huang, N.; Thilakavathy, K.; Lim, J.C.W.; Kumar, S.S.; Xiong, C. Glycyrrhizic Acid: A Natural Plant Ingredient as a Drug Candidate to Treat COVID-19. Front. Pharmacol. 2021, 12, 1740. [Google Scholar]
- Rocha, M.N.D.; Alves, D.R.; Marinho, M.M.; Morais, S.M.d.; Marinho, E.S. Virtual screening of citrus flavonoid tangeretin: A promising pharmacological tool for the treatment and prevention of Zika fever and COVID-19. J. Comput. Biophys. Chem. 2021, 20, 283–304. [Google Scholar] [CrossRef]
- Adem, S.; Eyupoglu, V.; Sarfraz, I.; Rasul, A.; Ali, M. Identification of potent COVID-19 main protease (Mpro) inhibitors from natural polyphenols: An in silico strategy unveils a hope against CORONA. Preprints 2020, 2020030333. [Google Scholar] [CrossRef] [Green Version]
- Tallei, T.E.; Tumilaar, S.G.; Niode, N.J.; Kepel, B.J.; Idroes, R.; Effendi, Y.; Sakib, S.A.; Emran, T.B. Potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and spike (S) glycoprotein inhibitors: A molecular docking study. Scientifica 2020, 2020, 6307457. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.; Bouziane, I.; Bouslama, Z.; Djemel, A. In-silico identification of potent inhibitors of COVID-19 main protease (Mpro) and angiotensin converting enzyme 2 (ACE2) from natural products: Quercetin, hispidulin, and cirsimaritin exhibited better potential inhibition than hydroxy-chloroquine against COVID-19 main protease active site and ACE2. ChemRxiv 2020. Version 1. [Google Scholar] [CrossRef]
- Murck, H. Symptomatic protective action of glycyrrhizin (licorice) in COVID-19 infection? Front. Immunol. 2020, 11, 1239. [Google Scholar] [CrossRef]
- Dabaghian, F.; Khanavi, M.; Zarshenas, M.M. Bioactive compounds with possible inhibitory activity of Angiotensin-Converting Enzyme-II; a gate to manage and prevent COVID-19. Med. Hypotheses 2020, 143, 109841. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-H.; Min, J.S.; Jeon, S.; Lee, J.; Kim, S.; Park, T.; Park, D.; Jang, M.S.; Park, C.M.; Song, J. Lycorine, a non-nucleoside RNA dependent RNA polymerase inhibitor, as potential treatment for emerging coronavirus infections. Phytomed 2021, 86, 153440. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, H.; Watashi, K.; Saso, W.; Shionoya, K.; Iwanami, S.; Hirokawa, T.; Shirai, T.; Kanaya, S.; Ito, Y.; Kim, K.S. Potential anti-COVID-19 agents, cepharanthine and nelfinavir, and their usage for combination treatment. iScience 2021, 24, 102367. [Google Scholar] [CrossRef] [PubMed]
- Gani, M.A.; Nurhan, A.D.; Maulana, S.; Siswodihardjo, S.; Shinta, D.W.; Khotib, J. Structure-based virtual screening of bioactive compounds from Indonesian medical plants against severe acute respiratory syndrome coronavirus-2. J. Adv. Pharm. Technol. Res. 2021, 12, 120–126. [Google Scholar] [PubMed]
- Shakya, A.; Chikhale, R.V.; Bhat, H.R.; Alasmary, F.A.; Almutairi, T.M.; Ghosh, S.K.; Alhajri, H.M.; Alissa, S.A.; Nagar, S.; Islam, M.A. Pharmacoinformatics-based identification of transmembrane protease serine-2 inhibitors from Morus Alba as SARS-CoV-2 cell entry inhibitors. Mol. Divers. 2021, 1–14. [Google Scholar] [CrossRef]
- Sawikowska, A. Meta-analysis of flavonoids with antiviral potential against coronavirus. Biom. Lett. 2020, 57, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ye, F.; Sun, Q.; Liang, H.; Li, C.; Li, S.; Lu, R.; Huang, B.; Tan, W.; Lai, L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J. Enzym. Inhib. Med. Chem. 2021, 36, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Remali, J.; Aizat, W.M. A review on plant bioactive compounds and their modes of action against coronavirus infection. Front. Pharmacol. 2020, 11, 589044. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Heng, W.; Wang, Y.; Qiu, J.; Wei, X.; Peng, S.; Saleem, S.; Khan, M.; Ali, S.S.; Wei, D.Q. In silico and in vitro evaluation of kaempferol as a potential inhibitor of the SARS-CoV-2 main protease (3CLpro). Phytother. Res. 2021, 35, 2841–2845. [Google Scholar] [CrossRef]
- Alagu Lakshmi, S.; Shafreen, R.M.B.; Priya, A.; Shunmugiah, K.P. Ethnomedicines of Indian origin for combating COVID-19 infection by hampering the viral replication: Using structure-based drug discovery approach. J. Biomol. Struct. Dyn. 2020, 39, 4594–4609. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.K.; Ali, D.; Alarifi, S.; Radhakrishnan, S.; Akbar, I. In silico molecular docking: Evaluation of coumarin based derivatives against SARS-CoV-2. J. Biomol. Struct. Dyn. 2020, 13, 1671–1677. [Google Scholar]
- Abd El-Aziz, N.M.; Shehata, M.G.; Eldin Awad, O.M.; El-Sohaimy, S.A. Inhibition of COVID-19 RNA-dependent RNA polymerase by natural bioactive compounds: Molecular docking analysis. Egypt. J. Chem. 2021, 64, 1989–2001. [Google Scholar]
- He, C.-L.; Huang, L.-Y.; Wang, K.; Gu, C.-J.; Hu, J.; Zhang, G.-J.; Xu, W.; Xie, Y.-H.; Tang, N.; Huang, A.-L. Identification of bis-benzylisoquinoline alkaloids as SARS-CoV-2 entry inhibitors from a library of natural products. Signal. Transduct. Target. Ther. 2021, 6, 1–3. [Google Scholar] [CrossRef]
- Jo, S.; Kim, S.; Kim, D.Y.; Kim, M.-S.; Shin, D.H. Flavonoids with inhibitory activity against SARS-CoV-2 3CLpro. J. Enzym. Inhib. Med. Chem. 2020, 35, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Muralikumar, V.; Ramakrishnamacharya, C.; Seshachalam, C. Technology, Inhibitory effect of phytochemicals from Azadirachta indica a juss. and Tinospora cordifolia (thunb.) miers against SARS-COV-2 mpro and spike protease—An in silico analysis. Int. J. Eng. Appl. Sci. 2020, 5, 303–319. [Google Scholar]
- Kumar, V.; Dhanjal, J.K.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (Mpro) of SARS-CoV-2 and inhibit its activity. J. Biomol. Struct. Dyn. 2020, 39, 3842–3854. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Siddiqui, S.; Ahmad, R.; Mehrotra, S.; Ahmad, B.; Srivastava, A. Exploring nature’s bounty: Identification of Withania somnifera as a promising source of therapeutic agents against COVID-19 by virtual screening and in silico evaluation. J. Biomol. Struct. Dyn. 2020, 1–51. [Google Scholar] [CrossRef]
- Javed, H.; Meeran, M.F.N.; Jha, N.K.; Ojha, S. Carvacrol, a Plant Metabolite Targeting Viral Protease (Mpro) and ACE2 in Host Cells Can Be a Possible Candidate for COVID-19. Front. Plant. Sci. 2020, 11, 601335. [Google Scholar] [CrossRef] [PubMed]
- Gurung, A.B.; Ali, M.A.; Lee, J.; Farah, M.A.; Al-Anazi, K.M. Unravelling lead antiviral phytochemicals for the inhibition of SARS-CoV-2 Mpro enzyme through in silico approach. Life Sci. 2020, 255, 117831. [Google Scholar] [CrossRef] [PubMed]
- Sabet, R.; Sisakht, M.; Emami, L.; Sabahi, Z. Comparison of COVID-19 Virus Main Protease Inhibition Activities of Phenolic Acids By Molecular Docking. Trends Pharmacol. Sci. 2021, 7, 117–126. [Google Scholar]
- Kulkarni, S.A.; Nagarajan, S.K.; Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J. Mol. Struct. 2020, 1221, 128823. [Google Scholar] [CrossRef]
- Jamali, N.; Soureshjani, E.H.; Mobini, G.-R.; Samare-Najaf, M.; Clark, C.C.; Saffari-Chaleshtori, J. Medicinal plant compounds as promising inhibitors of coronavirus (COVID-19) main protease: An in silico study. J. Biomol. Struct. Dyn. 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, A.A. Rutin as a promising inhibitor of main protease and other protein targets of Covid-19: In silico study. J. Nat. Prod. Commun. 2020, 15, 1934578X20953951. [Google Scholar] [CrossRef]
- Senthil Kumar, K.; Gokila Vani, M.; Wang, C.-S.; Chen, C.-C.; Chen, Y.-C.; Lu, L.-P.; Huang, C.-H.; Lai, C.-S.; Wang, S.-Y. Geranium and lemon essential oils and their active compounds downregulate angiotensin-converting enzyme 2 (ACE2), a SARS-CoV-2 spike receptor-binding domain, in epithelial cells. Plants 2020, 9, 770. [Google Scholar] [CrossRef]
- Ghosh, R.; Chakraborty, A.; Biswas, A.; Chowdhuri, S. Computer aided identification of potential SARS CoV-2 main protease inhibitors from diterpenoids and biflavonoids of Torreya nucifera leaves. J. Biomol. Struct. Dyn. 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Firdiana, E.R.; Renjana, E.; Ningrum, L.W.; Angio, M.H.; Nikmatullah, M.; Rizal, S. In Silico Study of the Active Compounds of Lindera aggregata (Sims) Kosterm as Anti-coronavirus. Curr. Nutr. Food. Sci. 2021, 17, 408–416. [Google Scholar] [CrossRef]
- Chikhale, R.V.; Sinha, S.K.; Patil, R.B.; Prasad, S.K.; Shakya, A.; Gurav, N.; Prasad, R.; Dhaswadikar, S.R.; Wanjari, M.; Gurav, S.S. In-silico investigation of phytochemicals from Asparagus racemosus as plausible antiviral agent in COVID-19. J. Biomol. Struct. Dyn. 2020, 39, 5033–5047. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zheng, W.; Li, M.; Huang, J.; Bao, S.; Xu, Q.; Ma, Z. Citrus fruits are rich in flavonoids for immunoregulation and potential targeting ACE2. Preprints 2020, 1–7. Available online: https://www.preprints.org/manuscript/202002.0313/v1 (accessed on 3 November 2021).
- Chen, Q.; Lan, H.-Y.; Peng, W.; Rahman, K.; Liu, Q.-C.; Luan, X.; Zhang, H. Pharmacology, Isatis indigotica: A review of phytochemistry, pharmacological activities and clinical applications. J. Pharm. Pharmacol. 2021, 73, 1137–1150. [Google Scholar] [CrossRef]
- Daglia, M.; Di Lorenzo, A.; Nabavi, S.F.; Talas, Z.S.; Nabavi, S.M. Polyphenols: Well beyond the antioxidant capacity: Gallic acid and related compounds as neuroprotective agents: You are what you eat! Curr. Pharm. Biotechnol. 2014, 15, 362–372. [Google Scholar] [CrossRef]
- Lucas, K.; Fröhlich-Nowoisky, J.; Oppitz, N.; Ackermann, M. Cinnamon and hop extracts as potential immunomodulators for severe COVID-19 cases. Front. Plant. Sci. 2021, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-H.; Huang, Y.-F.; Wang, W.-Y.; Yang, L.; Zhou, H.; Sang, Z. Analysis on the current quality standards of Chinese materia Medica used in COVID-19 prevention and treatment. Pharmacol. Res. 2020, 160, 105074. [Google Scholar] [CrossRef]
- Panyod, S.; Ho, C.-T.; Sheen, L.-Y. Dietary therapy and herbal medicine for COVID-19 prevention: A review and perspective. J. Tradit. Complement. Med. Mol. Divers. 2020, 10, 420–427. [Google Scholar] [CrossRef]
- Upreti, S.; Prusty, J.S.; Pandey, S.C.; Kumar, A.; Samant, M. Identification of novel inhibitors of angiotensin-converting enzyme 2 (ACE-2) receptor from Urtica dioica to combat coronavirus disease 2019 (COVID-19). Mol. Divers. 2021, 25, 1795–1809. [Google Scholar] [CrossRef]
- Lau, K.-M.; Lee, K.-M.; Koon, C.-M.; Cheung, C.S.-F.; Lau, C.-P.; Ho, H.-M.; Lee, M.Y.-H.; Au, S.W.-N.; Cheng, C.H.-K.; Bik-San Lau, C. Immunomodulatory and anti-SARS activities of Houttuynia cordata. J. Ethnopharmacol. 2008, 118, 79–85. [Google Scholar] [CrossRef]
- Wen, C.-C.; Shyur, L.-F.; Jan, J.-T.; Liang, P.-H.; Kuo, C.-J.; Arulselvan, P.; Wu, J.-B.; Kuo, S.-C.; Yang, N.-S.J. Traditional Chinese medicine herbal extracts of Cibotium barometz, Gentiana scabra, Dioscorea batatas, Cassia tora, and Taxillus chinensis inhibit SARS-CoV replication. J. Tradit. Complement. Med. 2011, 1, 41–50. [Google Scholar] [CrossRef] [Green Version]
- Shawky, E.; Nada, A.A.; Ibrahim, R.S. Potential role of medicinal plants and their constituents in the mitigation of SARS-CoV-2: Identifying related therapeutic targets using network pharmacology and molecular docking analyses. RSC Adv. 2020, 10, 27961–27983. [Google Scholar] [CrossRef]
- Pamukova-Michaelson, R.; Vodenicharova, A.; Mihaylov, C. Effect of Combined Therapies on Respiratory Diseases and Covid-19. Gen. Med. 2020, 22, 59–66. [Google Scholar]
- Khubber, S.; Hashemifesharaki, R.; Mohammadi, M.; Gharibzahedi, S.M.T. Garlic (Allium sativum L.): A potential unique therapeutic food rich in organosulfur and flavonoid compounds to fight with COVID-19. Nutr. J. 2020, 19, 1–3. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, B.; Lv, J.-T.; Sa, R.-N.; Zhang, X.-M.; Lin, Z.-J. The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence. Pharmacol. Res. 2020, 157, 104882. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.; Bag, A. Treatment of COVID-19 patients: Justicia adhatoda leaves extract is a strong remedy for COVID-19–Case report analysis and docking based study. ChemRxiv 2020, 1–7. [Google Scholar] [CrossRef]
- Sinha, S.K.; Prasad, S.K.; Islam, M.A.; Gurav, S.S.; Patil, R.B.; AlFaris, N.A.; Aldayel, T.S.; AlKehayez, N.M.; Wabaidur, S.M.; Shakya, A. Identification of bioactive compounds from Glycyrrhiza glabra as possible inhibitor of SARS-CoV-2 spike glycoprotein and non-structural protein-15: A pharmacoinformatics study. J. Biomol. Struct. Dyn. 2021, 39, 4686–4700. [Google Scholar] [CrossRef]
- Adhikari, B.; Marasini, B.P.; Rayamajhee, B.; Bhattarai, B.R.; Lamichhane, G.; Khadayat, K.; Adhikari, A.; Khanal, S.; Parajuli, N. Potential roles of medicinal plants for the treatment of viral diseases focusing on COVID-19: A review. Phytother. Res. 2021, 35, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Subbaiyan, A.; Ravichandran, K.; Singh, S.V.; Sankar, M.; Thomas, P.; Dhama, K.; Malik, Y.S.; Singh, R.K.; Chaudhuri, P. In silico molecular docking analysis targeting SARS-CoV-2 spike protein and selected herbal constituents. J. Pure Appl. Microbiol. 2020, 14, 989–998. [Google Scholar] [CrossRef]
- Seyran, M.; Takayama, K.; Uversky, V.N.; Lundstrom, K.; Palù, G.; Sherchan, S.P.; Attrish, D.; Rezaei, N.; Aljabali, A.A.; Ghosh, S. The structural basis of accelerated host cell entry by SARS-CoV-2. FEBS Jr. 2020, 288, 5010–5020. [Google Scholar] [CrossRef]
- Anand, K.; Ziebuhr, J.; Wadhwani, P.; Mesters, J.R.; Hilgenfeld, R.J.S. Coronavirus main proteinase (3CLpro) structure: Basis for design of anti-SARS drugs. Science 2003, 300, 1763–1767. [Google Scholar] [CrossRef] [Green Version]
- Lung, J.; Lin, Y.S.; Yang, Y.H.; Chou, Y.L.; Shu, L.H.; Cheng, Y.C.; Liu, H.T.; Wu, C.Y. The potential chemical structure of anti-SARS-CoV-2 RNA-dependent RNA polymerase. J. Med. Virol. 2020, 92, 693–697. [Google Scholar] [CrossRef]
- Nawrot-Hadzik, I.; Zmudzinski, M.; Matkowski, A.; Preissner, R.; Kęsik-Brodacka, M.; Hadzik, J.; Drag, M.; Abel, R. Reynoutria Rhizomes as a Natural Source of SARS-CoV-2 Mpro Inhibitors–Molecular Docking and In Vitro Study. Pharmaceuticals 2021, 14, 742. [Google Scholar] [CrossRef]
- Garg, S.; Anand, A.; Lamba, Y.; Roy, A. Molecular docking analysis of selected phytochemicals against SARS-CoV-2 M pro receptor. Vegetos 2020, 33, 766–781. [Google Scholar] [CrossRef] [PubMed]
- Suručić, R.; Tubić, B.; Stojiljković, M.P.; Djuric, D.M.; Travar, M.; Grabež, M.; Šavikin, K.; Škrbić, R. Computational study of pomegranate peel extract polyphenols as potential inhibitors of SARS-CoV-2 virus internalization. Mol. Cell. Biochem. 2021, 476, 1179–1193. [Google Scholar] [CrossRef]
- Murugan, N.A.; Pandian, C.J.; Jeyakanthan, J.S. Computational investigation on Andrographis paniculata phytochemicals to evaluate their potency against SARS-CoV-2 in comparison to known antiviral compounds in drug trials. J. Biomol. Struct. Dyn. 2021, 39, 4415–4426. [Google Scholar] [CrossRef] [PubMed]
- Saakre, M.; Mathew, D.; Ravisankar, V. Perspectives on plant flavonoid quercetin-based drugs for novel SARS-CoV-2. Beni-Suef Univ. J. Basic Appl. Sci. 2021, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Elgohary, S.; Elkhodiry, A.A.; Amin, N.S.; Stein, U.; El Tayebi, H.M. Thymoquinone: A tie-breaker in SARS-CoV2-infected cancer patients? Cells 2021, 10, 302. [Google Scholar] [CrossRef]
- van den Berg, D.F.; Te Velde, A.A. Severe COVID-19: NLRP3 inflammasome dysregulated. Front. Immunol. 2020, 11, 1580. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Anil, S.M.; Shalev, N.; Vinayaka, A.C.; Nadarajan, S.; Namdar, D.; Belausov, E.; Shoval, I.; Mani, K.A.; Mechrez, G.; Koltai, H. Cannabis compounds exhibit anti-inflammatory activity in vitro in COVID-19-related inflammation in lung epithelial cells and pro-inflammatory activity in macrophages. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Meeran, M.N.; Arunachalam, S.; Javed, H.; Sharma, C.; Hashiesh, H.M.; Goyal, S.N.; Jha, N.K.; Ojha, S. Can limonene be a possible candidate for evaluation as an agent or adjuvant against infection, immunity, and inflammation in COVID-19? Heliyon 2020, 7, e05703. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Samec, M.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Al-Ishaq, R.K.; Abotaleb, M.; Nosal, V.; Kajo, K.; Ashrafizadeh, M. Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed. Pharmacother. 2021, 138, 111430. [Google Scholar] [CrossRef] [PubMed]
- Balkrishna, A.; Solleti, S.K.; Verma, S.; Varshney, A. Application of humanized zebrafish model in the suppression of SARS-CoV-2 spike protein induced pathology by tri-herbal medicine coronil via cytokine modulation. Molecules 2020, 25, 5091. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yujing, S.; Jie, S.; Friedemann, T.; Zhenggang, T.; Lu, Y.; Yun, L.; Lv, Y.; Ronghua, Z.; Zihan, G. Shufeng Jiedu, a promising herbal therapy for moderate COVID-19: Antiviral and anti-inflammatory properties, pathways of bioactive compounds, and a clinical real-world pragmatic study. Phytomedicine 2021, 85, 153390. [Google Scholar]
- Khan, T.; Khan, M.A.; Ullah, N.; Nadhman, A.J.B.; Biotechnology, A. Therapeutic potential of medicinal plants against COVID-19: The role of antiviral medicinal metabolites. Biocatal. Agric. Biotechnol. 2020, 31, 101890. [Google Scholar] [CrossRef]
- Fakhri, S.; Nouri, Z.; Moradi, S.Z.; Farzaei, M.H. Astaxanthin, COVID-19 and immune response: Focus on oxidative stress, apoptosis and autophagy. Phytother. Res. 2020, 34, 2790–2792. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Bi, K.; He, Y.; Yan, W.; Yang, C.S.; Zhang, J. Potential protective mechanisms of green tea polyphenol EGCG against COVID-19. Trends Food Sci. Technol. 2021, 114, 11–24. [Google Scholar] [CrossRef]
- Du, A.; Zheng, R.; Disoma, C.; Li, S.; Chen, Z.; Li, S.; Liu, P.; Zhou, Y.; Shen, Y.; Liu, S. Epigallocatechin-3-gallate, an active ingredient of Traditional Chinese Medicines, inhibits the 3CLpro activity of SARS-CoV-2. Int. J. Biol. Macromol. 2021, 176, 1–12. [Google Scholar] [CrossRef]
- Jang, K.J.; Choi, S.H.; Yu, G.J.; Hong, S.H.; Chung, Y.H.; Kim, C.H.; Yoon, H.M.; Kim, G.Y.; Kim, B.W.; Choi, Y.H. Anti-inflammatory potential of total saponins derived from the roots of Panax ginseng in lipopolysaccharide-activated RAW 264.7 macrophages. Exp. Ther. Med. 2016, 11, 1109–1115. [Google Scholar] [CrossRef]
- Derouiche, S. Current Review on Herbal Pharmaceutical improve immune responses against COVID-19 infection. Res. J. Pharm. Dos. Technol. 2020, 12, 181–184. [Google Scholar] [CrossRef]
- Chowdhury, P.; Barooah, A.K. Tea bioactive modulate innate immunity: In perception to COVID-19 pandemic. Front. Immunol. 2020, 11, 590716. [Google Scholar] [CrossRef] [PubMed]
- Rishi, P.; Thakur, K.; Vij, S.; Rishi, L.; Singh, A.; Kaur, I.P.; Patel, S.K.; Lee, J.-K.; Kalia, V.C. Diet, gut microbiota and COVID-19. Indian J. Microbiol. 2020, 60, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Donma, M.M.; Donma, O. The effects of allium sativum on immunity within the scope of COVID-19 infection. Med. Hypotheses 2020, 144, 109934. [Google Scholar] [CrossRef] [PubMed]
- Brendler, T.; Al-Harrasi, A.; Bauer, R.; Gafner, S.; Hardy, M.L.; Heinrich, M.; Hosseinzadeh, H.; Izzo, A.A.; Michaelis, M.; Nassiri-Asl, M. Botanical drugs and supplements affecting the immune response in the time of COVID-19: Implications for research and clinical practice. Phytother. Res. 2021, 35, 3013–3031. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, D.-Q.; Wang, M.-C.; Chen, H.; Chen, L.; Liu, D.; Zhao, H.; Zhao, Y.-Y. Poricoic acid ZA, a novel RAS inhibitor, attenuates tubulo-interstitial fibrosis and podocyte injury by inhibiting TGF-β/Smad signaling pathway. Phytomedicine 2017, 36, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, D.-Q.; Chen, L.; Liu, D.; Zhao, H.; Zhang, Z.-H.; Vaziri, N.D.; Guo, Y.; Zhao, Y.-Y.; Cao, G. Novel RAS inhibitors poricoic acid ZG and poricoic acid ZH attenuate renal fibrosis via a Wnt/β-catenin pathway and targeted phosphorylation of smad3 signaling. J. Agric. Food. Chem. 2018, 66, 1828–1842. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yang, T.; Wang, M.-C.; Chen, D.-Q.; Yang, Y.; Zhao, Y.-Y. Novel RAS inhibitor 25-O-methylalisol F attenuates epithelial-to-mesenchymal transition and tubulo-interstitial fibrosis by selectively inhibiting TGF-β-mediated Smad3 phosphorylation. Phytomedicine 2018, 42, 207–218. [Google Scholar] [CrossRef]
- Jiang, C.; Shao, Q.; Jin, B.; Gong, R.; Zhang, M.; Xu, B. Tanshinone IIA attenuates renal fibrosis after acute kidney injury in a mouse model through inhibition of fibrocytes recruitment. Biomed. Res. Int. 2015, 2015, 867140. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Bo, Y.; Shen, W.; Tan, J.; Jia, Z.; Xu, C.; Li, F. Leonurine ameliorates kidney fibrosis via suppressing TGF-β and NF-κB signaling pathway in UUO mice. Int. Immunopharmacol. 2015, 25, 406–415. [Google Scholar] [CrossRef]
- Ma, Z.; Tang, Y.; Zhong, L.; Yu, K.; He, L. Anti-fibrosis and relative mechanism of salvianolic acid A on rat model with renal fibrosis. Int. J. Clin. Exp. Med. 2016, 9, 12713–12720. [Google Scholar]
- Yuan, X.P.; Liu, L.S.; Fu, Q.; Wang, C.X. Effects of Ligustrazine on Ureteral Obstruction-induced Renal Tubulointerstitial Fibrosis. Phytother. Res. 2012, 26, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.-Z.; Guo, X.-T.; Chen, J.-W.; Zhao, Y.; Cong, X.; Jiang, Z.-L.; Cao, R.-F.; Cui, K.; Gao, S.-S.; Tian, W.-R. Saikosaponin-D attenuates heat stress-induced oxidative damage in LLC-PK1 cells by increasing the expression of anti-oxidant enzymes and HSP72. Am. J. Chin. Med. 2014, 42, 1261–1277. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.P.; He, X.S.; Wang, C.X.; Liu, L.S.; Fu, Q. Triptolide attenuates renal interstitial fibrosis in rats with unilateral ureteral obstruction. Nephrology 2011, 16, 200–210. [Google Scholar] [CrossRef]
- Shokeir, A.A.; Barakat, N.; Hussein, A.M.; Awadalla, A.; Harraz, A.; Khater, S.; Hemmaid, K.; Kamal, A.I. Activation of Nrf2 by ischemic preconditioning and sulforaphane in renal ischemia/reperfusion injury: A comparative experimental study. Physiol. Res. 2015, 64, 313. [Google Scholar] [CrossRef] [PubMed]
- Paixão, J.; Dinis, T.C.; Almeida, L.M. Malvidin-3-glucoside protects endothelial cells up-regulating endothelial NO synthase and inhibiting peroxynitrite-induced NF-kB activation. Chem. Biol. Interact. 2012, 199, 192–200. [Google Scholar] [CrossRef]
- Jang, Y.J.; Park, B.; Lee, H.-W.; Park, H.J.; Koo, H.J.; Kim, B.O.; Sohn, E.-H.; Um, S.H.; Pyo, S. Sinigrin attenuates the progression of atherosclerosis in ApoE−/− mice fed a high-cholesterol diet potentially by inhibiting VCAM-1 expression. Chem. Biol. Interact. 2017, 272, 28–36. [Google Scholar] [CrossRef]
- Skemiene, K.; Rakauskaite, G.; Trumbeckaite, S.; Liobikas, J.; Brown, G.C.; Borutaite, V. Anthocyanins block ischemia-induced apoptosis in the perfused heart and support mitochondrial respiration potentially by reducing cytosolic cytochrome c. Int. J. Biochem. Cell Biol. 2013, 45, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Nagi, M.N.; Al-Shabanah, O.A.; Hafez, M.M.; Sayed-Ahmed, M.M. Thymoquinone supplementation attenuates cyclophosphamide-induced cardiotoxicity in rats. J. Biochem. Mol. Toxicol. 2011, 25, 135–142. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, S.; Zou, X.; Jing, Y.; Yang, R.; Li, S.; Wang, F. Ginsenoside Rb1 improves cardiac function and remodeling in heart failure. Exp. Anim. 2017, 66, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Paiva, L.; Lima, E.; Neto, A.I.; Baptista, J. Angiotensin I-converting enzyme (ACE) inhibitory activity of Fucus spiralis macroalgae and influence of the extracts storage temperature—A short report. J. Pharm. Biomed. Anal. 2016, 131, 503–507. [Google Scholar] [CrossRef]
- Agrawal, K.; Melliou, E.; Li, X.; Pedersen, T.L.; Wang, S.C.; Magiatis, P.; Newman, J.W.; Holt, R.R. Oleocanthal-rich extra virgin olive oil demonstrates acute anti-platelet effects in healthy men in a randomized trial. J. Funct. Foods 2017, 36, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yang, S.; Du, J. Protective effects of berberine on isoproterenol-induced acute myocardial ischemia in rats through regulating HMGB1-TLR4 axis. Evid. Based Complement. Altern. Med. 2014, 2014, 849783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Zhang, Y.; Zhu, M.; Zhang, Q.; Wang, X.; Wang, Y.; Zhang, J.; Li, J.; Yang, L.; Liu, J. Resveratrol attenuates myocardial ischemia/reperfusion injury through up-regulation of vascular endothelial growth factor B. Free Radic. Biol. Med. 2016, 101, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Ma, M.; Fei, X.; Zhang, Y.; Gong, F.; Fang, M. Glycyrrhizin attenuates hepatic ischemia-reperfusion injury by suppressing HMGB1-dependent GSDMD-mediated kupffer cells pyroptosis. Int. Immunopharmacol. 2019, 68, 145–155. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Dai, L.-H.; Wu, Y.-H.; Yu, X.-P.; Zhang, Y.-Y.; Guan, R.-F.; Liu, T.; Zhao, J.J.B.; Bulletin, P. Evaluation of hepatocyteprotective and anti-hepatitis B virus properties of Cichoric acid from Cichorium intybus leaves in cell culture. Biol. Pharm. Bull. 2014, 37, 1214–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruck, R.; Ashkenazi, M.; Weiss, S.; Goldiner, I.; Shapiro, H.; Aeed, H.; Genina, O.; Helpern, Z.; Pines, M. Prevention of liver cirrhosis in rats by curcumin. Liver Int. 2007, 27, 373–383. [Google Scholar] [CrossRef]
- Kang, M.-C.; Kang, S.-M.; Ahn, G.; Kim, K.-N.; Kang, N.; Samarakoon, K.W.; Oh, M.-C.; Lee, J.-S.; Jeon, Y.-J. Protective effect of a marine polyphenol, dieckol against carbon tetrachloride-induced acute liver damage in mouse. Environ. Toxicol. Pharmacol. 2013, 35, 517–523. [Google Scholar] [CrossRef]
- Ma, J.Q.; Ding, J.; Zhao, H.; Liu, C.M. Puerarin attenuates carbon tetrachloride-induced liver oxidative stress and hyperlipidaemia in mouse by JNK/c-Jun/CYP7A1 pathway. Basic Clin. Pharmacol. Toxicol. 2014, 115, 389–395. [Google Scholar] [CrossRef]
- Calland, N.; Sahuc, M.-E.; Belouzard, S.; Pène, V.; Bonnafous, P.; Mesalam, A.A.; Deloison, G.; Descamps, V.; Sahpaz, S.; Wychowski, C. Polyphenols inhibit hepatitis C virus entry by a new mechanism of action. J. Virol. 2015, 89, 10053–10063. [Google Scholar] [CrossRef] [Green Version]
- Rasool, M.K.; Sabina, E.P.; Ramya, S.R.; Preety, P.; Patel, S.; Mandal, N.; Mishra, P.P.; Samuel, J. Pharmacology, Hepatoprotective and antioxidant effects of gallic acid in paracetamol-induced liver damage in mice. J. Pharm. Ther. 2010, 62, 638–643. [Google Scholar]
- Sun, H.; Che, Q.-M.; Zhao, X.; Pu, X.-P. Antifibrotic effects of chronic baicalein administration in a CCl4 liver fibrosis model in rats. Eur. J. Pharmacol. 2010, 631, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-F.; Zhang, Y.-Q.; Fan, S.-H.; Zhuang, J.; Zheng, Y.-L.; Lu, J.; Wu, D.-M.; Shan, Q.; Hu, B. Troxerutin protects against 2, 2′, 4, 4′-tetrabromodiphenyl ether (BDE-47)-induced liver inflammation by attenuating oxidative stress-mediated NAD+-depletion. J. Hazard. Mater. 2015, 283, 98–109. [Google Scholar] [CrossRef]
- Ren, J.; Fan, C.; Chen, N.; Huang, J.; Yang, Q. Resveratrol pretreatment attenuates cerebral ischemic injury by upregulating expression of transcription factor Nrf2 and HO-1 in rats. Neurochem. Res. 2011, 36, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.M.; Mohamed, W.R.; Omar, H.A. A neuroprotective role of kaempferol against chlorpyrifos-induced oxidative stress and memory deficits in rats via GSK3β-Nrf2 signaling pathway. Pestic. Biochem. Physiol. 2018, 152, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Lyu, M.; Wang, Y.; He, S.; Liu, X.; Ni, J.; Li, L.; Fan, G.; Han, J.; Gao, X. Ginkgo Flavonol Glycosides or Ginkgolides Tend to Differentially Protect Myocardial or Cerebral Ischemia–Reperfusion Injury via Regulation of TWEAK-Fn14 Signaling in Heart and Brain. Front. Pharmacol. 2019, 10, 735. [Google Scholar] [CrossRef]
- Song, Z.; Han, S.; Pan, X.; Gong, Y.; Wang, M. Pharmacology, Pterostilbene mediates neuroprotection against oxidative toxicity via oestrogen receptor α signalling pathways. J. Pharm. Pharmacol. 2015, 67, 720–730. [Google Scholar] [CrossRef]
- Chen, P.; Lin, X.; Yang, C.-H.; Tang, X.; Chang, Y.-W.; Zheng, W.; Luo, L.; Xu, C.; Chen, Y.-H. Study on chemical profile and neuroprotective activity of Myrica rubra leaf extract. Molecules 2017, 22, 1226. [Google Scholar] [CrossRef] [Green Version]
- Pegorini, S.; Braida, D.; Verzoni, C.; Guerini-Rocco, C.; Consalez, G.G.; Croci, L.; Sala, M. Capsaicin exhibits neuroprotective effects in a model of transient global cerebral ischemia in Mongolian gerbils. Br. J. Pharmacol. 2005, 144, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Koh, S.S.; Ooi, S.C.-Y.; Lui, N.M.-Y.; Qiong, C.; Ho, L.T.-Y.; Cheah, I.K.-M.; Halliwell, B.; Herr, D.R.; Ong, W.-Y. Effect of ergothioneine on 7-ketocholesterol-induced endothelial injury. Neuromol. Med. 2021, 23, 184–198. [Google Scholar] [CrossRef]
- Wang, J.; Li, P.; Qin, T.; Sun, D.; Zhao, X.; Zhang, B. Protective effect of epigallocatechin-3-gallate against neuroinflammation and anxiety-like behavior in a rat model of myocardial infarction. Brain Behav. 2020, 10, e01633. [Google Scholar] [CrossRef]
- Mammana, S.; Cavalli, E.; Gugliandolo, A.; Silvestro, S.; Pollastro, F.; Bramanti, P.; Mazzon, E. Could the combination of two non-psychotropic cannabinoids counteract neuroinflammation? Effectiveness of cannabidiol associated with cannabigerol. Medicina 2019, 55, 747. [Google Scholar] [CrossRef] [Green Version]
- Amann, K.; Boor, P.; Wiech, T.; Singh, J.; Vonbrunn, E.; Knöll, A.; Hermann, M.; Büttner-Herold, M.; Daniel, C.; Hartmann, A. COVID-19 effects on the kidney. Pathology 2021, 42, 183–187. [Google Scholar]
- Chan, L.; Chaudhary, K.; Saha, A.; Chauhan, K.; Vaid, A.; Zhao, S.; Paranjpe, I.; Somani, S.; Richter, F.; Miotto, R. AKI in hospitalized patients with COVID-19. J. Am. Soc. Nephrol. 2021, 32, 151–160. [Google Scholar] [CrossRef]
- Benedetti, C.; Waldman, M.; Zaza, G.; Riella, L.V.; Cravedi, P. COVID-19 and the kidneys: An update. Front. Med. (Lausanne) 2020, 7, 423. [Google Scholar] [CrossRef]
- Sfera, A.; Osorio, C.; Jafri, N.; Diaz, E.L.; Campo Maldonado, J.E. Intoxication with endogenous angiotensin II: A COVID-19 hypothesis. Front. Immunol. 2020, 11, 1472. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, T.M.; Lakkappa, N.; Lazartigues, E. ADAM17-mediated shedding of inflammatory cytokines in hypertension. Front. Pharmacol. 2020, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.P.; Bourne, T.D.; Wilson, J.D.; Saqqa, O.; Sharshir, M.A. Collapsing glomerulopathy in a patient with COVID-19. Kidney Int. Rep. 2020, 5, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Kissling, S.; Rotman, S.; Gerber, C.; Halfon, M.; Lamoth, F.; Comte, D.; Lhopitallier, L.; Sadallah, S.; Fakhouri, F. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020, 98, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yang, M.; Wan, C.; Yi, L.-X.; Tang, F.; Zhu, H.-Y.; Yi, F.; Yang, H.-C.; Fogo, A.B.; Nie, X. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Li, Z.; Wu, M.; Yao, J.; Guo, J.; Liao, X.; Song, S.; Li, J.; Duan, G.; Zhou, Y.; Wu, X. Caution on kidney dysfunctions of COVID-19 patients. MedRxiv 2020. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Tang, Y.; Huang, X.-R.; Yu, X.; Lan, H.-Y. Inflammatory stress in SARS-COV-2 associated Acute Kidney Injury. Int. J. Biol. Sci. 2021, 17, 1497. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Gomes, M.; Kruglov, A.; Durek, P.; Heinrich, F.; Tizian, C.; Heinz, G.A.; Pascual-Reguant, A.; Du, W.; Mothes, R.; Fan, C. SARS-CoV-2 in severe COVID-19 induces a TGF-β-dominated chronic immune response that does not target itself. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Zhang, M.; Cen, H.; Wu, Y.-F.; Lu, Z.; Lu, F.; Liu, X.-S.; Lan, H.-Y. Quercetin as a potential treatment for COVID-19-induced acute kidney injury: Based on network pharmacology and molecular docking study. PLoS ONE 2021, 16, e0245209. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.Z.; Wang, C.; Deng, C.; Zhong, X.; Yan, Y.; Luo, Y.; Lan, H.Y.; He, T.; Wang, L. Quercetin protects against cisplatin-induced acute kidney injury by inhibiting Mincle/Syk/NF-κB signaling maintained macrophage inflammation. Phytother. Res. 2020, 34, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Plebani, M.J.C.C.; Medicine, L. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. 2020, 58, 1131–1134. [Google Scholar] [CrossRef] [Green Version]
- Arentz, M.; Yim, E.; Klaff, L.; Lokhandwala, S.; Riedo, F.X.; Chong, M.; Lee, M. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington State. JAMA 2020, 323, 1612–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential effects of coronaviruses on the cardiovascular system: A review. JAMA Cardiol. 2020, 5, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Bansal, M. Cardiovascular disease and COVID-19. Diabetes Metab. Syndr. 2020, 14, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tian, J.; Xu, Y.; Li, C.; Meng, X.; Fu, F. Protective effect of RA on myocardial infarction-induced cardiac fibrosis via AT1R/p38 MAPK pathway signaling and modulation of the ACE2/ACE ratio. J. Agric. Food Chem. 2016, 64, 6716–6722. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pang, W.; He, C.; Li, Y.; Jiang, Y.; Guo, C. Blueberry anthocyanin-enriched extracts attenuate fine particulate matter (PM2. 5)-induced cardiovascular dysfunction. J. Agric. Food Chem. 2017, 65, 87–94. [Google Scholar] [CrossRef]
- Escudero-López, B.; Berná, G.; Ortega, Á.; Herrero-Martín, G.; Cerrillo, I.; Martín, F.; Fernández-Pachón, M.-S. Consumption of orange fermented beverage reduces cardiovascular risk factors in healthy mice. Food Chem. Toxicol. 2015, 78, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Sa-Ngiamsuntorn, K.; Suksatu, A.; Pewkliang, Y.; Thongsri, P.; Kanjanasirirat, P.; Manopwisedjaroen, S.; Charoensutthivarakul, S.; Wongtrakoongate, P.; Pitiporn, S.; Chaopreecha, J. Anti-SARS-CoV-2 activity of Andrographis paniculata extract and its major component Andrographolide in human lung epithelial cells and cytotoxicity evaluation in major organ cell representatives. J. Nat. Prod. 2021, 84, 1261–1270. [Google Scholar] [CrossRef]
- Wang, B.; Kovalchuk, A.; Li, D.; Rodriguez-Juarez, R.; Ilnytskyy, Y.; Kovalchuk, I.; Kovalchuk, O. In search of preventive strategies: Novel high-CBD Cannabis sativa extracts modulate ACE2 expression in COVID-19 gateway tissues. Aging 2020, 12, 22425–22444. [Google Scholar] [PubMed]
- Orlando, G.; Chiavaroli, A.; Adorisio, S.; Delfino, D.V.; Brunetti, L.; Recinella, L.; Leone, S.; Zengin, G.; Acquaviva, A.; Angelini, P. Unravelling the Phytochemical Composition and the Pharmacological Properties of an Optimized Extract from the Fruit from Prunus mahaleb L.: From Traditional Liqueur Market to the Pharmacy Shelf. Molecules 2021, 26, 4422. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Srivastava, A.K.; Ray, U.; Tripathi, P.P. Is the collapse of the respiratory center in the brain responsible for respiratory breakdown in COVID-19 patients? ACS Chem. Neurosci. 2020, 11, 1379–1381. [Google Scholar] [CrossRef]
- Al-Ramadan, A.; Rabab’h, O.; Shah, J.; Gharaibeh, A. Acute and post-acute neurological complications of COVID-19. Neurol. Int. 2021, 13, 102–119. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Li, M.; Wang, M.; Zhou, Y.; Chang, J.; Xian, Y.; Wang, D.; Mao, L.; Jin, H.; Hu, B. Acute cerebrovascular disease following COVID-19: A single center, retrospective, observational study. Stroke Vasc. Neurol. 2020, 5, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Scullen, T.; Keen, J.; Mathkour, M.; Dumont, A.S.; Kahn, L. Coronavirus 2019 (COVID-19)–associated encephalopathies and cerebrovascular disease: The New Orleans experience. World Neurosurg. 2020, 141, e437–e446. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.F.; Ho, Y.-C. SARS-CoV-2: A storm is raging. J. Clin. Investig. 2020, 130, 2202–2205. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Ortiz, C.; Méndez-Guerrero, A.; Rodrigo-Rey, S.; San Pedro-Murillo, E.; Bermejo-Guerrero, L.; Gordo-Mañas, R.; de Aragón-Gómez, F.; Benito-León, J. Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology 2020, 95, e601–e605. [Google Scholar] [CrossRef] [Green Version]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lan Psychy. 2021, 8, 416–427. [Google Scholar] [CrossRef]
- Rajkumar, R.P. Ayurveda and COVID-19: Where psychoneuroimmunology and the meaning response meet. Brain. Behav. Immun. 2020, 87, 8–9. [Google Scholar] [CrossRef]
- Kempuraj, D.; Thangavel, R.; Kempuraj, D.D.; Ahmed, M.E.; Selvakumar, G.P.; Raikwar, S.P.; Zaheer, S.A.; Iyer, S.S.; Govindarajan, R.; Chandrasekaran, P.N. Neuroprotective effects of flavone luteolin in neuroinflammation and neurotrauma. Biofactors 2021, 47, 190–197. [Google Scholar] [CrossRef]
- Theoharides, T.; Conti, P. COVID-19 and multisystem inflammatory syndrome, or is it mast cell activation syndrome. J. Biol. Regul. Homeost. Agents 2020, 34, 1633–1636. [Google Scholar] [PubMed]
- Martinez, M.A.; Franco, S. Impact of COVID-19 in liver disease progression. Hepatol. Commun. 2021, 5, 1138–1150. [Google Scholar] [CrossRef]
- Bertolini, A.; van de Peppel, I.P.; Bodewes, F.; Moshage, H.; Fantin, A.; Farinati, F.; Fiorotto, R.; Jonker, J.W.; Strazzabosco, M.; Verkade, H. Abnormal liver function tests in patients with COVID-19: Relevance and potential pathogenesis. Hepatology 2020, 72, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Phipps, M.M.; Barraza, L.H.; LaSota, E.D.; Sobieszczyk, M.E.; Pereira, M.R.; Zheng, E.X.; Fox, A.N.; Zucker, J.; Verna, E.C. Acute liver injury in COVID-19: Prevalence and association with clinical outcomes in a large US cohort. Hepatology 2020, 72, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, L.; Wang, F.-S. Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 2020, 5, 428–430. [Google Scholar] [CrossRef]
- Zhao, B.; Ni, C.; Gao, R.; Wang, Y.; Yang, L.; Wei, J.; Lv, T.; Liang, J.; Zhang, Q.; Xu, W.; et al. Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020, 11, 771–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, S.; Liu, H.; Li, W.; Lin, F.; Jiang, L.; Li, X.; Xu, P.; Zhang, L.; Zhao, L. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020, 73, 807–816. [Google Scholar] [CrossRef]
- Goldman, J.D.; Lye, D.C.; Hui, D.S.; Marks, K.M.; Bruno, R.; Montejano, R.; Spinner, C.D.; Galli, M.; Ahn, M.-Y.; Nahass, R.G.; et al. Remdesivir for 5 or 10 days in patients with severe Covid-19. N. Engl. J. Med. 2020, 383, 1827–1837. [Google Scholar] [CrossRef]
- Hernandez, A.V.; Roman, Y.M.; Pasupuleti, V.; Barboza, J.J.; White, C.M. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: A living systematic review. Ann. Intern. Med. 2020, 173, 287–296. [Google Scholar] [CrossRef]
- Hodgman, M.J.; Garrard, A.R. A review of acetaminophen poisoning. Crit. Care Clin. 2012, 28, 499–516. [Google Scholar] [CrossRef]
- Li, H.; Hu, Y.; Tang, H.; Li, S.; Ding, H.; Zhai, S.; Zhao, R. The Potential of Glycyrrhizinate in the Management of COVID-19: A Systematic Review of the Efficacy and Safety of Glycyrrhizin Preparations in the Treatment of SARS and MERS. Am. J. Chin. Med. 2020, 48, 1539–1552. [Google Scholar] [CrossRef] [PubMed]
- Liao, F.L.; Peng, D.H.; Chen, W.; Hu, H.N.; Tang, P.; Liu, Y.Y.; Luo, Y.; Yao, T. Evaluation of serum hepatic enzyme activities in different COVID-19 phenotypes. J. Med. Virol. 2021, 93, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Xiang, X.; Chen, W.; Yang, Z.; Hu, W.; Qu, H.; Liu, J. Efficacy of diammonium glycyrrhizinate combined with vitamin C for treating hospitalized COVID-19 patients: A retrospective, observational study. QJM Int. J. Med. 2021. [CrossRef]
- Roshdy, W.H.; Rashed, H.A.; Kandeil, A.; Mostafa, A.; Moatasim, Y.; Kutkat, O.; Abo Shama, N.M.; Gomaa, M.R.; El-Sayed, I.H.; El Guindy, N.M. EGYVIR: An immunomodulatory herbal extract with potent antiviral activity against SARS-CoV-2. PLoS ONE 2020, 15, e0241739. [Google Scholar] [CrossRef]
- Storz, M.A. Lifestyle Adjustments in Long-COVID Management: Potential Benefits of Plant-Based Diets. Curr. Nutr. Rep. 2021, 1–12. [Google Scholar] [CrossRef]
- Girija, P.; Sivan, N. Ayurvedic treatment of COVID-19/SARS-CoV-2: A case report. J. Ayurveda Integr. Med. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Fu, F.; Ye, H.; Gao, H.; Tan, B.; Wang, R.; Lin, N.; Qin, L.; Chen, W. Chinese herbal Huo-Gu formula for the treatment of steroid-associated osteonecrosis of femoral head: A 14-year follow-up of convalescent SARS patients. J. Orthop. Translat. 2020, 23, 122–131. [Google Scholar] [CrossRef] [PubMed]



| Medicinal Plants | Plant Active Compound | Antiviral Activity | References |
|---|---|---|---|
| Citrus aurantium | Hesperidin | Inhibition of main protease (Mpro) | [32] |
| Camellia sinensis | Epigallocatechin gallate | Inhibition of spike glycoprotein (S-protein) | [33] |
| Linaria vulgaris | Pectolinarin | Inhibition of spike glycoprotein and Mpro | [33] |
| Rhus succedanea | Rhoifolin | Inhibition of Mpro | [33] |
| Coffea arabica | Epigallocatechin gallate | Inhibition of Mpro | [33] |
| Cirsium japonicum | Cirsimaritin | Inhibits against Mpro | [34] |
| Glycyrrhiza glabra | Glycyrrhizin | Reduces the level of TMPRSS2 | [35] |
| Acalypha australis | Emodin | ACE-II Inhibitors | [36] |
| Citrus reticulata | Nobeletin | ACE-II Inhibitors | [36] |
| Lycoris squamigera | Lycorine | Inhibition of RdRP activity | [37] |
| Stephania cepharantha | Cepharanthine | Inhibition of viral-target binding | [38] |
| Citrus bergamia | Naringenin | ACE-II Inhibitors | [36] |
| Justicia procumbens | Justicidin D | Inhibition of RdRP and spike protein | [39] |
| Oroxylum indicum | Baicalin | ACE-II Inhibitors | [36] |
| Curcuma longa | Curcumin | Inhibits against Mproactivity | [34] |
| Morus alba | Moralbanone | Inhibition of SARS-3CLpro | [40] |
| Linum usitatissimum | Herbacetin | Inhibits the activity of 3CLpro | [41] |
| Maclura tinctoria | Morin | Inhibition of Mpro and S-protein | [33] |
| Cirsium chanroenicum | Pectolinarin | Inhibition of Mpro and S-protein | [33] |
| Scutellaria baicalensis | Scutellarin | Inhibition of SARS-3CLpro | [42] |
| Taraxacum officinale | Epitaraxerol | Inhibits viral activity | [43] |
| Ginkgo biloba | Kaempferol | Inhibition of SARS-3CLpro activity | [44] |
| Berberis buxifolia | Berberine | Targets SARS-Cov-2 Mpro activity | [45] |
| Phyllostachys nigra | Vitexine | Targets SARS-Cov-2 Mproactivity | [45] |
| Toddalia asiatica (Linn.) Lam. | Toddacoumaquinone | Targets SARS-Cov-2 Mproactivity | [46] |
| Olea europaea | Oleuropein | RdRP inhibitors | [47] |
| Vitis vinifera | Resveratrol | RdRP inhibitors | [47] |
| Caryophyllales sp. | Bisbenzylisoquinoline | Suppressing viral entry | [48] |
| Oroxylum indicum | Baicelin | Inhibits the activity of 3CLpro | [49] |
| Tinospora cordifolia | Cordifolide A | Inhibition of Mpro and S-protein | [50] |
| Withania somnifera | Withanone | Inhibits the active protease Mpro | [51] |
| Withania somnifera | Withanolide B | Inhibits papain like receptor and ACE-II receptor | [52] |
| Origanum vulgare | Carvacrol | Inhibits main protease (Mpro) and SARS-CoV-2 entry | [53] |
| Caesalpinia minax. | Bonducellpin D | Inhibition of Mpro | [54] |
| Salvia officinalis L. | Rosemarinic acid | Inhibits the activity of 3CLpro | [55] |
| Thymus vulgaris L. | Thymol | Inhibits spike glycoprotein | [56] |
| Nepeta cataria | Pulegone | Inhibits spike glycoprotein | [56] |
| Glycyrrhiza glabra | Glabiridin | Inhibits the main protease | [57] |
| Fagopyrum esculentum | Rutin | Inhibition of the RNA dependent polymerase | [58] |
| Cymbopogon winterianus | Citronellol | ACE-II Inhibitors | [59] |
| Torreya nucifera L. | Bilobetin, amentoflavonone | Inhibition of Mpro | [60] |
| Lindera aggregate | Linderane and linderalactone | Inhibition of 3CLpro and spike protein | [61] |
| Asparagus racemosus | Asparoside-C, Asparoside-D and Asparoside -F | Inhibition of spike protein and nsp 15 | [62] |
| Citrus reticulata | Hesperitin, hesperidin | Inhibition of 3CLpro | [63] |
| Isatis indigotica | Hesperitin, hesperidin, rutin, phaitanthrin D | Inhibition of 3CLpro | [64,65] |
| Cinnamomum zeylanicum | Apigenin, geranylated flavonoids | Reduction in transcription factor NF-κB activation | [66] |
| Panax ginseng | Polysaccharides | Immunomodulatory properties | [67,68] |
| Urtica dioica | β-sitosterol, tannic acid | Inhibitor of ACE-II receptor | [69] |
| Houttuynia cordata | Bavachinin, cosmosilin, | SARS-3CLproinhibition, RdRP | [70] |
| Cibotium barometz | Neohesperidin | Inhibition SARS-3CL protease | [71] |
| Hibiscus sabdariffa L. | Anthocyanin. cyanidine, anastatin A | Inhibition SARS-3CL protease | [72] |
| Salvia officinalis | Luteolin, caffeic acid, | Inhibits viral replication | [73] |
| Allium sativum | Alliin, diayl disulfide, allicin, quercetin | Decreasing of viral infection rate and interacts with Mpro | [74] |
| Mentha piperita | Terpenoids and menthol | Inhibition of acute respiratory infection | [75] |
| Morus alba | Moralbanone, mulberroside C, eudraflavone B | Inhibition of viral replication | [40] |
| Justicia adhatoda | Anisotine, vasicoline, pemirolast | Inhibition of RdRP | [76] |
| Natural Compounds | Source | Pathology | Mechanism | Effective Concentration | References |
|---|---|---|---|---|---|
| Kidney damage | |||||
| Poricoic acid ZA | Poriacocos | Renal fibrosis (RF) | Inhibits Smad2/3 phosphorylation | 10 µM | [106] |
| poricoic acid ZG | Poriacocos | RF | Inhibits Smad3 and alter TGF-β/Smad signaling | 10 µM | [107] |
| poricoic acid ZH | Poriacocos | RF | Inhibits Wnt or β-catenin signaling | 10 µM | [107] |
| 25-O-methylalisol | Alisma rhizoma | RF | Inhibits Wnt or β-catenin signaling | 10 µM | [108] |
| Tanshinone IIA | Salvia multiorrhiza | Acute kidney injury | Inhibits TGF-β1 and MCP-1 expression | 15 mg/kg | [109] |
| Leonurine | Leonurus spp. | RF | Inhibits TGF-β/Smad pathway | 50 mg/kg/day | [110] |
| Salvianolic acid A | Salvia multiorrhiza | RF | Inhibits TGF-β1/Smad signaling and inflammatory cytokines | 17.1 mg/kg | [111] |
| Ligustrazine | Ligusticum striatum | RF | Inhibits TGF-β pathway | 80 mg/kg | [112] |
| Saikosaponin-D | Bupleurum falcatum | Oxidative stress | Increases antioxidant proteins CAT, SOD1, HSP72 and GPx-1 | 3 µg/mL | [113] |
| Triptolide | Tripterygium wilfordii | RF | Decreases TGF-β and MCP-1 expression | 0.6 mg/kg | [114] |
| Sulforaphane | Brassica oleracea | Renal ischemia | Increases the level of Nrf2 and NQO-1 | 500 µg/kg | [115] |
| Cardiovascular disease | |||||
| Malvidin-3-glucoside | Vitis spp. | Endothelial dysfunction | Reduces iNOS, COX-2, IL-6 through inhibition of NF-kβ activation | 25 µM | [116] |
| Sinigrin | Brassica spp. | Atheresclerosis | Attenuates VCAM-1, ICAM-1, CCL2, CCL5 expression and reduces LDH, LDL concentration in serum | 10 mg/kg | [117] |
| Delphinidin | Delphinium spp. | Heart ischemia | Rapid reduction of cytochrome c | 40 µM | [118] |
| Cyanidin | Vaccinium spp. | Heart ischemia | Rapid reduction of cytochrome c | 40 µM | [118] |
| Thymoquinone | Nigella sativa | Cardiac failure | Decreases oxidative and nitrosative stress | 200 mg/kg | [119] |
| Ginsenoside Rb1 | Panax spp. | Cardiac failure | Reduces the level of ANF, β-MHC, Ang II, ACE, AT1 and enhances translocation of GLUT4 to plasma membrane | 35 mg/kg, 70 mg/kg | [120] |
| Phlorotannin | Fucus spiralis | High blood pressure. | Inhibits ACE | 200 µg/mL | [121] |
| Oleocanthal | Olea europaea | Atherosclerosis | Inhibition of platelet aggregation mediated inflammation | 40 mL of extra virgin olive oil | [122] |
| Barberine | Berberis spp. | Myocardial ischemia | Decreases CK-MB, LDH, TNF-α, IL-6 and regulate HMGB1-TLR4 axis | 30 mg/kg, 60 mg/kg | [123] |
| Resveratrol | Vitis spp. | Myocardial ischemia | Promotes VEGF-B/ antioxidant signaling pathway | 10 µM | [124] |
| Liver injury | |||||
| Glycyrrhizin | Glycyrrhiza glabra | Hepatic ischemia-reperfusion | Inhibits Gasdermin D-mediated pyroptotic cell death of Kupffer cells | 100 uM | [125] |
| Chicoric acid | Cichorium intybus | Hepatitis B | Block viral protein and DNA replication | 100 µg/mL | [126] |
| Curcumin | Curcuma longa | Liver cirrhosis | Reduces oxidative stress | 300 mg/kg/day | [127] |
| Dieckol | Ecklonia cava | Carbon tetrachloride induced liver damage | Upregulates antioxidant enzymes | 25 mg/kg | [128] |
| Puerarin | Pueraria lobata | Carbon tetrachloride induced liver damage | Regulates JNK/c-Jun/CYP7A1 Pathway | 400 mg/kg | [129] |
| Delphinidin | Delphinium | Hepatitis C | Impairs viral attachment to cell | 100 µM | [130] |
| Gallic acid | Fragaria ananassa | Paracetamol induced liver damage | Decreases TNF-α and lipid peroxidation levels | 100 mg/kg | [131] |
| Baicalein | Scutellaria baicalensis | Carbon tetrachloride induced liver damage | Decreases AST and ALT levels | 80 mg/kg | [132] |
| Troxerutin | Styphnolobium japonicum | Liver inflammation | Reduces oxidative stress mediated NAD+-depletion | 150 mg/kg/day | [133] |
| Neurological disorder | |||||
| Resveratrol | Vitis spp. | Cerebral ishchemia | Enhances Nrf2 and HO-1 expression | 30 mg/kg | [134] |
| Kaempferol | Brassica oleracea | Oxidative stress induced neurotoxicity | Inhibits GSK3β and enhance Nrf2 expression | 21 mg/kg | [135] |
| Ginkgolide | Ginkgo biloba | Cerebral ishchemia-repurfusion injury | Elevates the TNF related weak initiator of apoptosis (TWEAK) ligand | 2.5 mL/kg | [136] |
| Pterostilbene | Pterocarpus marsupium | Oxidative stress induced neurotoxicity | Acts as estrogen like compound to activate estrogen receptor-α (ER-α) mediated signaling | 10 nM | [137] |
| Myricanol | Myrica rubra | Oxidative stress induced neurotoxicity | Enhances Nrf2 expression | 50 mg/mL | [138] |
| Capsaicin | Capsicum annuum | Cerebral ischemia | Regulates transient receptor potential channel vanilloid subfamily member 1 (VR1) | 0.2 mg/kg | [139] |
| Ergothioneine | Pleurotus ostreatus | Endothelial Injury | Decreases IL-8, IL-6, TNF-α, COX2 expression | 10 µM | [140] |
| Epigallocatechin-3-gallate | Camellia sinensis | Neuroinflammation | Alleviates the STAT3 and IL-6 levels | 50 mg/mL | [141] |
| Cannabigerol& cannabidiol | Cannabis sativa | Neuroinflammation | Reduces NF-kB activation and increase Nrf2 levels. | 5 µM | [142] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, P.; Bose, S.; Srivastava, A.K.; Chaudhary, A.A.; Lall, R.; Prasad, S. Jeopardy of COVID-19: Rechecking the Perks of Phytotherapeutic Interventions. Molecules 2021, 26, 6783. https://doi.org/10.3390/molecules26226783
Saha P, Bose S, Srivastava AK, Chaudhary AA, Lall R, Prasad S. Jeopardy of COVID-19: Rechecking the Perks of Phytotherapeutic Interventions. Molecules. 2021; 26(22):6783. https://doi.org/10.3390/molecules26226783
Chicago/Turabian StyleSaha, Priyanka, Subhankar Bose, Amit Kumar Srivastava, Anis Ahmad Chaudhary, Rajiv Lall, and Sahdeo Prasad. 2021. "Jeopardy of COVID-19: Rechecking the Perks of Phytotherapeutic Interventions" Molecules 26, no. 22: 6783. https://doi.org/10.3390/molecules26226783