Nicotine in Combination with SARS-CoV-2 Affects Cells Viability, Inflammatory Response and Ultrastructural Integrity
Abstract
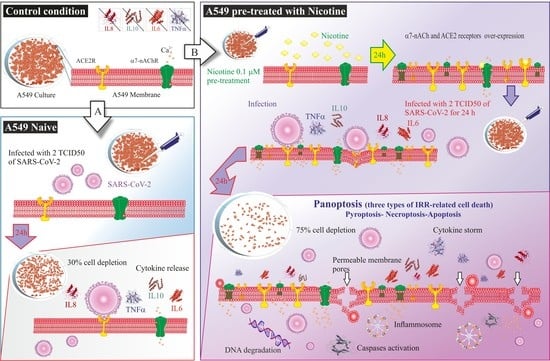
:1. Introduction
2. Results
2.1. Evaluation of Cytokine Release in Control, Nicotine-Treated, and SARS-CoV-2 Infected Cells
2.2. Cell Cytotoxicity
2.3. Ultrastructural Changes at TEM
3. Discussion
4. Materials and Methods
4.1. Cells and Chemicals
4.2. SARS-CoV-2 Culture
4.3. Cell Viability Assay
4.4. Markers of Inflammation
4.5. Transmission Electron Microscopy
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Costa, L.B.; Perez, L.G.; Palmeira, V.A.; Macedo, E.C.T.; Ribeiro, V.T.; Lanza, K.; Simoes, E.S.A.C. Insights on SARS-CoV-2 Molecular Interactions with the Renin-Angiotensin System. Front. Cell Dev. Biol. 2020, 8, 559841. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Sa, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Goncalves, A.V.; Perucello, D.B.; An-drade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef]
- del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Luo, M.; Liu, J.; Jiang, W.; Yue, S.; Liu, H.; Wei, S. IL-6 and CD8+ T cell counts combined are an early predictor of in-hospital mortality of patients with COVID-19. JCI Insight 2020, 5, 139024. [Google Scholar] [CrossRef]
- Gustine, J.N.; Jones, D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2021, 191, 4–17. [Google Scholar] [CrossRef]
- Pelaia, C.; Tinello, C.; Vatrella, A.; de Sarro, G.; Pelaia, G. Lung under attack by COVID-19-induced cytokine storm: Patho-genic mechanisms and therapeutic implications. Ther. Adv. Respir. Dis. 2020, 14, 1753466620933508. [Google Scholar] [CrossRef]
- The Lancet Respiratory Medicine. COVID-19, smoking, and cancer: A dangerous liaison. Lancet Respir. Med. 2021, 9, 937. [Google Scholar] [CrossRef]
- Clancy, L.; Gallus, S.; Leung, J.; Egbe, C.O. Tobacco and COVID-19: Understanding the science and policy implications. Tob. Induc. Dis. 2020, 18, 105. [Google Scholar] [CrossRef]
- Besaratinia, A. COVID-19: A pandemic converged with global tobacco epidemic and widespread vaping-state of the evidence. Carcinogenesis 2021, 42, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Herbec, A.; Brown, J.; Jackson, S.E.; Kale, D.; Zatoński, M.; Garnett, C.; Chadborn, T.; Shahab, L. Perceived risk factors for severe COVID-19 symptoms and their association with health behaviours: Findings from the HEBECO study. Acta Psychol. 2022, 222, 103458. [Google Scholar] [CrossRef] [PubMed]
- Krebs, N.M.; D’Souza, G.; Bordner, C.; Allen, S.I.; Hobkirk, A.L.; Foulds, J.; Yingst, J.M. COVID-19 Vaccination Uptake and Hesitancy Among Current Tobacco Users. Tob. Use Insights 2021, 14, 1179173X211068027. [Google Scholar] [CrossRef]
- Changeux, J.P.; Amoura, Z.; Rey, F.A.; Miyara, M. A nicotinic hypothesis for COVID-19 with preventive and therapeutic implications. C. R. Biol. 2020, 343, 33–39. [Google Scholar] [PubMed]
- Hopkinson, N.S.; Rossi, N.; El-Sayed_Moustafa, J.; Laverty, A.A.; Quint, J.K.; Freidin, M.; Visconti, A.; Murray, B.; Modat, M.; Ourselin, S.; et al. Current smoking and COVID-19 risk: Results from a population symptom app in over 2.4 million people. Thorax 2021, 76, 714–722. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. NCT04598594 Evaluation of the Efficacy of Nicotine Patches in SARS-CoV2 (COVID-19) Infection in Intensive Care Unit Patients (NICOVID-REA). Available online: https://clinicaltrials.gov/ct2/show/NCT04598594 (accessed on 1 July 2022).
- ClinicalTrials.gov. NCT04583410 Efficacy of Nicotine in Preventing COVID-19 Infection (NICOVID-PREV). Available online: https://clinicaltrials.gov/ct2/show/NCT04583410 (accessed on 1 July 2022).
- ClinicalTrials.gov. NCT04608201 Evaluation of the Efficacy of Nicotine Patches in SARS-CoV2 (COVID-19) Infection in Hospitalized Patients (NICOVID). Available online: https://clinicaltrials.gov/ct2/show/NCT04608201 (accessed on 1 July 2022).
- Maggi, F.; Rosellini, A.; Spezia, P.G.; Focosi, D.; Macera, L.; Lai, M.; Pistello, M.; de Iure, A.; Tomino, C.; Bonassi, S.; et al. Nicotine upregulates ACE2 expression and increases competence for SARS-CoV-2 in human pneumocytes. ERJ Open Res. 2021, 7, 00713-2020. [Google Scholar] [CrossRef]
- Ogando, N.S.; Dalebout, T.J.; Zevenhoven-Dobbe, J.C.; Limpens, R.W.; van der Meer, Y.; Caly, L.; Druce, J.; de Vries, J.J.C.; Kikkert, M.; Bárcena, M.; et al. SARS-coronavirus-2 replication in Vero E6 cells: Replication kinetics, rapid adaptation and cytopathology. J. Gen. Virol. 2020, 101, 925–940. [Google Scholar] [CrossRef]
- Russo, P.; Bonassi, S.; Giacconi, R.; Malavolta, M.; Tomino, C.; Maggi, F. COVID-19 and smoking: Is nicotine the hidden link? Eur. Respir. J. 2020, 55, 2001116. [Google Scholar] [CrossRef]
- Lupacchini, L.; Maggi, F.; Tomino, C.; de Dominicis, C.; Mollinari, C.; Fini, M.; Bonassi, S.; Merlo, D.; Russo, P. Nicotine Changes Airway Epithelial Phenotype and May Increase the SARS-CoV-2 Infection Severity. Molecules 2020, 26, 101. [Google Scholar] [CrossRef]
- Leung, J.M.; Yang, C.X.; Sin, D.D. COVID-19 and nicotine as a mediator of ACE-2. Eur. Respir. J. 2020, 55, 2001261. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.M.; Sin, D.D. Smoking, ACE-2 and COVID-19: Ongoing controversies. Eur. Respir. J. 2020, 56, 2001759. [Google Scholar] [CrossRef] [PubMed]
- Lieber, M.; Todaro, G.; Smith, B.; Szakal, A.; Nelson-Rees, W. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer 1976, 17, 62–70. [Google Scholar] [CrossRef] [PubMed]
- McGowan, S.E. The Formation of Pulmonary Alveoli. In The Lung, 2nd ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 65–84. [Google Scholar] [CrossRef]
- Dittmar, M.; Lee, J.S.; Whig, K.; Segrist, E.; Li, M.; Kamalia, B.; Castellana, L.; Ayyanathan, K.; Cardenas-Diaz, F.L.; Morrisey, E.E.; et al. Drug repurposing screens reveal cell-type-specific entry pathways and FDA-approved drugs active against SARS-CoV-2. Cell Rep. 2021, 35, 108959. [Google Scholar] [CrossRef]
- Kleymenov, D.A.; Bykonia, E.N.; Popova, L.I.; Mazunina, E.P.; Gushchin, V.A.; Kolobukhina, L.V.; Burgasova, O.A.; Kruzhkova, I.S.; Kuznetsova, N.A.; Shidlovskaya, E.V.; et al. A Deep Look Into COVID-19 Severity Through Dynamic Changes in Blood Cytokine Levels. Front. Immunol. 2021, 12, 771609. [Google Scholar] [CrossRef]
- Li, Q.; Zhou, X.D.; Kolosov, V.P.; Perelman, J.M. Nicotine reduces TNF-alpha expression through an alpha7 nA-ChR/MyD88/NF-kB pathway in HBE16 airway epithelial cells. Cell Physiol. Biochem. 2011, 27, 605–612. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Salvi, V.; Nguyen, H.O.; Sozio, F.; Schioppa, T.; Gaudenzi, C.; Laffranchi, M.; Scapini, P.; Passari, M.; Barbazza, I.; Tiberio, L.; et al. SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8. JCI Insight 2021, 6, e150542. [Google Scholar] [CrossRef]
- Pan, P.; Shen, M.; Yu, Z.; Ge, W.; Chen, K.; Tian, M.; Xiao, F.; Wang, Z.; Wang, J.; Jia, Y.; et al. SARS-CoV-2 N protein promotes NLRP3 inflammasome activation to induce hyperinflammation. Nat. Commun. 2021, 12, 4664. [Google Scholar] [CrossRef]
- Mitoma, H.; Hanabuchi, S.; Kim, T.; Bao, M.; Zhang, Z.; Sugimoto, N.; Liu, Y.-J. The DHX33 RNA Helicase Senses Cytosolic RNA and Activates the NLRP3 Inflammasome. Immunity 2013, 39, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christgen, S.; Zheng, M.; Kesavardhana, S.; Karki, R.; Malireddi, R.K.S.; Banoth, B.; Place, D.E.; Briard, B.; Sharma, B.R.; Tuladhar, S.; et al. Identification of the PANoptosome: A Molecular Platform Triggering Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Lee, E.; Place, D.; Samir, P.; Mavuluri, J.; Sharma, B.R.; Balakrishnan, A.; Malireddi, R.S.; Geiger, R.; Zhu, Q.; et al. IRF8 Regulates Transcription of Naips for NLRC4 Inflammasome Activation. Cell 2018, 173, 920–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-alpha and IFN-gamma Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef]
- Vora, S.M.; Lieberman, J.; Wu, H. Inflammasome activation at the crux of severe COVID-19. Nat. Rev. Immunol. 2021, 21, 694–703. [Google Scholar] [CrossRef]
- Singh, G.B.; Kshirasagar, N.; Patibandla, S.; Puchchakayala, G.; Koka, S.; Boini, K.M. Nicotine instigates podocyte injury via NLRP3 inflammasomes activation. Aging 2019, 11, 12810–12821. [Google Scholar] [CrossRef]
- Fischer, F.A.; Chen, K.W.; Bezbradica, J.S. Posttranslational and Therapeutic Control of Gasdermin-Mediated Pyroptosis and Inflammation. Front. Immunol. 2021, 12, 661162. [Google Scholar] [CrossRef]
- Huang, H.-R.; Cho, S.J.; Harris, R.M.; Yang, J.; Bermejo, S.; Sharma, L.; de la Cruz, C.S.; Xu, J.-F.; Stout-Delgado, H.W. RIPK3 Activates MLKL-mediated Necroptosis and Inflammasome Signaling during Streptococcus Infection. Am. J. Respir. Cell Mol. Biol. 2021, 64, 579–591. [Google Scholar] [CrossRef]
- Gonzalez-Rubio, J.; Navarro-Lopez, C.; Lopez-Najera, E.; Lopez-Najera, A.; Jimenez-Diaz, L.; Navarro-Lopez, J.D.; Najera, A. Cytokine Release Syndrome (CRS) and Nicotine in COVID-19 Patients: Trying to Calm the Storm. Front. Immunol. 2020, 11, 1359. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sansone, L.; de Iure, A.; Cristina, M.; Belli, M.; Vitiello, L.; Marcolongo, F.; Rosellini, A.; Macera, L.; Spezia, P.G.; Tomino, C.; et al. Nicotine in Combination with SARS-CoV-2 Affects Cells Viability, Inflammatory Response and Ultrastructural Integrity. Int. J. Mol. Sci. 2022, 23, 9488. https://doi.org/10.3390/ijms23169488
Sansone L, de Iure A, Cristina M, Belli M, Vitiello L, Marcolongo F, Rosellini A, Macera L, Spezia PG, Tomino C, et al. Nicotine in Combination with SARS-CoV-2 Affects Cells Viability, Inflammatory Response and Ultrastructural Integrity. International Journal of Molecular Sciences. 2022; 23(16):9488. https://doi.org/10.3390/ijms23169488
Chicago/Turabian StyleSansone, Luigi, Antonio de Iure, Mario Cristina, Manuel Belli, Laura Vitiello, Federica Marcolongo, Alfredo Rosellini, Lisa Macera, Pietro Giorgio Spezia, Carlo Tomino, and et al. 2022. "Nicotine in Combination with SARS-CoV-2 Affects Cells Viability, Inflammatory Response and Ultrastructural Integrity" International Journal of Molecular Sciences 23, no. 16: 9488. https://doi.org/10.3390/ijms23169488