Abstract
Data sources This review article scrutinised 16 clinical studies (clinical trials and observational studies) concerning coronavirus disease of 2019 (COVID-19). Additionally, 18 guidelines about the COVID-19 were reviewed and the key points were represented in this study.
Study selection The review included human trials, in-vitro studies, review articles, and credible news reports about severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and COVID-19 complications, treatment guidelines, management strategies, and epidemiological features. There were no exclusion criteria reported in this review and the included information was in English and Chinese languages.
Data extraction and synthesis A descriptive review of the literature was conducted, taking a comprehensive approach. The paper consisted of three main parts: introduction, presentation, and management. The introduction part presented basic information about the SARS-CoV-2, its evolution and transmission, and the course of disease. The presentation section introduced the signs and symptoms, diagnosis, high risk groups, and complications of COVID-19. Eventually, some evidence was presented about the prevention, medical management, and measuring responses to the treatments in the management section.
Results Based on the results of this study, non-pharmaceutical interventions, including strict social isolation and distancing measures, might reduce the spread of the SARS-CoV-2 by nearly 99.3 percent (reproduction number mitigating from 406 to 2.5 in 30 days). In the supportive management section, monitoring vital signs and neonatal feeding were stated as the most important factors to consider. For symptomatic neonates, medical management and intervention were mentioned as essential. It was claimed that for adults with mild infection, the best option would be home quarantine with further medical monitoring or hospitalisation if required. The following sequence was also suggested as early supportive therapy and monitoring: intravenous fluid administration, oxygen therapy, and application of corticosteroids. Management of critical patients with critical COVID-19 included admission to intensive care unit, use of continuous positive airway pressure and bi-level positive airway pressure in certain circumstances, endotracheal intubation, invasive mechanical ventilation, extracorporeal membrane oxygenation, and fluid resuscitation and vasopressors. Additionally, this study suggested oseltamivir, iopinavir, remdesivir, chloroquine, baricitinib, ruxolitinib, and fedratinib as possible drugs to help manage COVID-19. A soaring c-reactive protein level and decreased albumin content in the blood were reported to be associated with a deteriorating status in COVID-19 patients. To keep the number of exposures to a minimum, two separate viral clearance tests taken at least 24 hours apart, were stated as necessary laboratory results before the discharge of patients with COVID-19.
Conclusions The study warns about possible exponential spread of COVID-19 and proposes to adhering to tighter restrictions of social distancing. Besides the clinical guidelines presented within the study, it also encourages further up-to-date and evidence-based management guidelines for patients with COVID-19.
Similar content being viewed by others
A commentary on
Nicola M, O'Neill N, Sohrabi C, Khan M, Agha M, Agha M.
Evidence Based Management Guideline for the COVID-19 Pandemic - Review Article. Int J Surg 2020; DOI: 10.1016/j.ijsu.2020.04.001.

GRADE rating
Commentary
After the appearance of coronavirus disease of 2019 (COVID-19), several countries have been facing detrimental effects, especially in the healthcare field. The number of the coronavirus cases is reported to be 4,964,032 with a mortality rate of 323,483 individuals to date.1 Accordingly, many clinical and epidemiological guidelines have been published to effectively address the problem. This study conducted a comprehensive review of the literature and further provided a management guideline for COVID-19 patients. Fig. 1 is illustrative of the concepts underpinning the guideline.
The aim of the study was stated to offer evidence-based review of current practice. However, the strategy of the data search is not transparent and the methods of identification are not identified. No explicit criteria were declared. Also, no methodologic validity assessment or weighting of the citations were reported. This review article did, however, achieve its goal of presenting basic information about COVID-19 and suggesting an evidence-based clinical management guideline.
The first part of the study introduced severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as a member of the coronaviridae family which affects the lower respiratory tract of humans. Angiotensin-converting enzyme 2 (ACE2) receptors appear to be the mode of entry into healthy cells. The incubation time of the disease was estimated to be 5.2 days within which the SARS-CoV-2 could be transmitted by respiratory droplets, bodily fluids, fecal-oral contact, direct contact, or through environmental surfaces. It was also stated that no vertical transmission of the virus occurs during pregnancy. Median time for recovery from the onset of symptoms was claimed to be approximately 2 weeks in mild cases and 3 to 6 weeks in severely or critically unwell individuals.
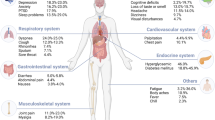
In the second part, comorbidities were reported to be associated with more susceptibility to severe infection in COVID-19 patients. Thereafter, healthcare workers with regular contact with COVID-19 patients, were in second place regarding risk of severe disease. As a result, healthcare workers with higher risks like increased age or chronic respiratory diseases were advised not to be involved in direct care provision for COVID-19 patients. A pooled estimation of the data showed that fever (92.8%), cough (69.8%), dyspnoea (34.5%), myalgia (27.7%), pharyngalgia (17.4%), headache (7.2%), diarrhoea (6.1%), sore throat (5.1%), and rhinorrhoea (4%) are the most prevalently shown symptoms in adult COVID-19 patients. Finally, a mortality rate of 1% to 2% was reported for the disease.
The last section of the study focused on presenting a clinical guideline for the disease. The authors deemed non-pharmaceutical interventions (NPIs) to be immensely effective in decreasing the reproduction number of COVID-19. After describing different types of NPIs, the adaptive triggering strategy was introduced as an effective way to regulate the social distancing policies in the absence of a vaccine. Lastly, a guideline for the medical management of COVID-19 patients was presented. Monitoring the vital signs, neonatal feeding, and medical intervention (in symptomatic neonates) were considered to be necessary. In adults with severe acute respiratory infection (SARI), the supportive management was defined as follows: intravenous fluid administration, oxygen therapy, and use of corticosteroids. Since corticosteroids might be indicated for other conditions, they should be administered cautiously. Management of critical COVID-19 patients included admission to intensive care unit (ICU), non-invasive ventilation (NIV), endotracheal intubation, invasive mechanical ventilation, extracorporeal membrane oxygenation, and fluid resuscitation and vasopressors. Also, a new shift in the consensus about using continuous positive airway pressure (CPAP) and bi-level positive airway pressure (BiPAP) were specified with certain indications as follows: as a ceiling of care option; attempting to avoid intubation; and attempting to aid extubation. A guide on how to calculate the positive end-expiratory pressure (PEEP) with the help of fraction of inspired oxygen (FiO2) was described. This was elicited from the 2000 Acute Respiratory Distress Syndrome Network (ARDSNet) trial.2 This strategy could be used to achieve an arterial oxygen saturation (SpO2) greater than 90%. In adults, fluid resuscitation should be administered as 250-500ml crystalloid fluid boluses over 15-30 min followed by assessment for fluid overload after each bolus and vasopressors might be used if septic shock persists despite fluid resuscitation. During fluid resuscitation, norepinephrine is the vasopressor of choice in adults, which could be supplemented by epinephrine or vasopressin to maintain mean arterial pressure ≥ 65 mmHg. Subsequently, some drugs have been proposed by this review for the medical management of COVID-19. Remdesivir and chloroquine were the first two of them which act by virus inhibition and cellular entry inhibition, respectively. Another way to decrease the damages imposed by SARS-CoV-2 is to decrease the inflammatory cytokines. Baricitinib, ruxolitinib, and fedratinib were introduced as three anti-inflammatory agents targeting JAK-STAT signalling pathway. A double lung transplant surgery was reported as an example of operative management of COVID-19 which was conducted on 29 February 2020. This might be considered as a new line of treatment for severe cases of COVID-19 in the future. It is stated that the response of patients to supportive treatment cannot currently be measured, however, a viral clearance of two respiratory tract specimens, obtained at least 24 hours apart, is suggested prior to patient discharge from the hospital.
Other clinical and epidemiological guidelines are also available and overall, the data presented in this study were in accordance with the existing literature.3,4 Providing clinicians with regularly updated and evidence-based guidelines is an imperative step to give the patients the best treatment options available. Although one might see the current pace of publications related to COVID-19 as peculiarly high, it is absolutely essential to be able to achieve a more robust consensus on the matter. These protocols and guidelines can eventually shape and direct the public health policies towards a better path which will lead to the best possible outcomes regarding the prevention, treatment, and management of the COVID-19 pandemic.
References
Coronavirus Update (Live): 4964032 coronavirus cases; 323483 deaths [Internet]. 2020. Available from: https://www.worldometers.info/coronavirus/ (accessed May 2020).
Network A R D S. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301-1308.
WHO. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected: interim guidance, 25 January 2020. World Health Organization; 2020.
Guan W-j, Ni Z-y, Hu Y et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708-20.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shamsoddin, E. A COVID-19 pandemic guideline in evidence-based medicine. Evid Based Dent 21, 71–73 (2020). https://doi.org/10.1038/s41432-020-0105-7
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41432-020-0105-7
This article is cited by
-
Prevention and control of COVID-19 by primary health care facilities in China: a field-survey-based qualitative study in three typical cities
BMC Health Services Research (2022)