Abstract
Remdesivir therapy has been declared as efficient in the early stages of Covid-19. Of the 339 patients (males 55.8%, age 71(59;77) years) with a detectable viral load, 140 were treated with remdesivir (of those 103 in the ICU and 57 immunosuppressed) and retrospectively compared with 199 patients (of those 82 in the ICU and 28 immunosuppressed) who were denied therapy due to advanced Covid-19. The viral load was estimated by detecting nucleocapsid antigen in serum (n = 155, median 217(28;1524)pg/ml), antigen in sputum (n = 18, COI 18(4.6;32)), nasopharyngeal antigen (n = 44, COI 17(8;35)) and the real-time PCR (n = 122, Ct 21(18;27)). After adjustment for confounders, patients on remdesivir had better 12-month survival (HR 0.66 (0.44;0.98), p = 0.039), particularly when admitted to the ICU (HR 0.49 (0.29;0.81), p = 0.006). For the immunocompromised patients, the difference did not reach statistical significance (HR 0.55 (0.18;1.69), p = 0.3). The other most significant confounders were age, ICU admission, mechanical ventilation, leukocyte/lymphocyte ratio, admission creatinine and immunosuppression. The impact of monoclonal antibodies or previous vaccinations was not significant. Despite frequent immune suppression including haemato-oncology diseases, lymphopenia, and higher inflammatory markers in the remdesivir group, the results support remdesivir administration with respect to widely available estimates of viral load in patients with high illness severity.
Similar content being viewed by others
Introduction
The adenosine triphosphate analogue remdesivir blocks the activity of viral polymerase and the build-up of the mRNA chain. The approved indication is for incipient forms of viral pneumonitis with a need for oxygen administration. The usual dosing scheme for severe coronavirus disease is 600 mg within 5 days (200 mg on the first day, followed by 100 mg daily), an abridged three-day dosing scheme has been approved for less severe forms in high-risk patients1,2,3,4,5.
The widely applied indication criteria for remdesivir were based on the duration of symptoms (less than 7 days), PCR positivity for SARS-CoV-2, full unlimited care with the expected ensuing quality of life corresponding to the Frailty Score 1–66,7. The risk factors supporting the indication have been lymphopenia, haemato-oncologic disease, vasculitis, obesity, diabetes, hypertension, and chronic obstructive pulmonary disease8,9,10,11. The drug should not be given to patients on high-flow nasal oxygen (HFNO) or mechanical ventilation due to anticipated advanced disease without a significant impact on therapeutic outcome7,12,13,14,15. Another relative contraindication is renal failure with respect to the result of a translational research in apes, which has not been fully validated in humans16,17.
Trials on remdesivir therapy in Covid-19 included patients with positive qualitative PCR testing and clinical symptoms within a time limit of several days. Therapy was declared to be more effective in the early stages of the disease and was thus denied in advanced forms of Covid-19 with respiratory failure or patients admitted to intensive care7,18,19,20.
However, the published data also alludes to the superior effect of virostatic therapy under the condition of high viral load21,22,23 that, in hospitalised symptomatic patients, correlates with morbidity and mortality24,25,26. This viral load can be estimated by the semiquantitative detection of SARS-CoV-2 antigen (specific nucleoproteins) using fluorescent immunochromatography of the sputum/nasal cavity, quantitative detection of serum nucleocapsid antigen27 and real-time PCR detection using the cycle threshold (Ct) to determine its positivity. Ct less than 28–30 has been correlated with a load of living virus and with the positivity of most of the quality antigen testing28. In contrast, Ct above 34 has not shown the presence of a living viral load, may indicate an early stage of the disease or represent a residual positivity after a previous viral load that was not monitored in symptomatic patients28,29,30,31.
The coronavirus mutations (beta, delta) detected during the course of the pandemic showcased prolonged replication times that often exceeded 3–5 days of the incubation period plus 7 days of clinical symptoms32,33,34. These prolonged replication times were typically reported in the immunocompromised and in patients with severe symptoms35,36,37. Thus, patients with fulminant courses of Covid-19 associating with admissions to intensive care may have high viral load and plasmatic viraemia, yet these patients should be excluded from remdesivir administration potentially due to a rapid need for invasive ventilatory support and, possibly even the ECMO therapy38,39.
The hypothesis of this retrospective study was that a long-term benefit of remdesivir in the most severe forms of Covid-19 might have associated with the presence of an active viral load, and this, to date, has been poorly validated in relation to widely available clinical testing. Patients treated with remdesivir according to the estimates of viral load using antigen tests or real-time PCR were compared with similarly positive patients where the drug was denied. The reasons why the drug was not administered despite the presence of a viral load were justified by the guidelines, for example, based on symptoms lasting for more than 7 days from the first positive test and due to advanced illness severity. The endpoints of the study were the short and long-term outcome data evaluated with confounding factors for the overall population and collected in two major Prague university hospitals. In addition, the sub-analysis of the intensive care patients and patients with immunocompromise (including oncology and haemato-oncologic patients) were aimed to clarify an impact of therapy in patients with the viral load and high illness severity. Results supporting more efficient remdesivir administration were expected with respect to a widely available semi-quantitative estimate of viral load. This research may potentially better justify antiviral therapy in the advanced stages of disease and serve as a basis for a prospective multicentric study.
Methods
In this retrospective study we compared patients with an indication for remdesivir based on the presence of a viral load with patients where the virostatic therapy was contraindicated. To underpin our hypothesis, we included Covid-19 patients in whom remdesivir therapy was withheld due to current guidelines and with, in parallel, the presence of a viral load detected by available laboratory tests, as controls.
In addition to the primary analysis the defined subgroups were patients with similar high illness severity, e.g., ICU patients, and patients with an immune deficit. These patients had history of immunosuppressive therapy including combined immunosuppression, with recent or ongoing oncology treatment including haemato-oncology therapy and on chronic dialysis.
The endpoints of the study were the short and long-term outcomes adjusted for anamnestic, diagnostic, and therapeutic factors. The comparisons of outcomes were assessed in patient subgroups with high illness severity, e.g., ICU patients, and patients with immunosuppression.
The patients were identified from the hospital database of laboratory results and patients who tested positive (implicating a presence of viral load, e.g., an abundance of living coronavirus either in blood or in the mucosal specimens) were included. The tests used were quantitative detection of a nucleocapsid antigen (NAg) in serum (SARS-CoV-2 Antigen Quantitative Assay Kit, Biohit Healthcare (Hefei) Co., ELISA, cut-off: 2.97 pg/ml, from 26.02. 2021 till 21.09. 2021 and SARS-CoV-2 Antigen Quantitative Test, Biohit Healthcare (Hefei) Co., fluorescence immunochromatography test, cut-off 8.92 pg/ml, after 22.09. 2021), semi-quantitative SARS-CoV-2 antigen (specific nucleoproteins) detection by fluorescent immune-chromatography in sputum/nasopharyngeal samples (cut-off-index (COI) 1, Standard F Covid-19 Ag FIA, SD Biosensor) and real-time PCR using cycle threshold for positivity (Ct) in sputum/nasopharyngeal samples. Patients included in the study had a diagnosis of a viral load confirmed by one of the aforementioned tests. The cut off for the NAg in serum was set to > 20 pg/ml, to COI > 10 for the immunofluorescent antigen in either the nasopharyngeal or sputum samples, and the cycle threshold of the real-time PCR on the nasopharyngeal or sputum samples had to be lower than 28. Antigen tests have been available for routine use since 2021 and the patients were recruited over the period of 16 months between the 1st of February 2021 and 31st of May 2022.
Patients who tested positive for the presence of a viral load were divided into a group treated with remdesivir and a group where the drug was denied/withheld due to delayed admission after an initial positive test or due to the advanced illness severity where the therapy was deemed futile.
The patient´s data included age, gender, body mass index (BMI), length-of-stay in the ICU and in the hospital. The acute physiology and chronic health evaluation score IV (APACHE IV) was calculated as a mortality predictor in every ICU patient on admission. Illness severity was characterised by the sequential organ failure score (SOFA), the degree of respiratory insufficiency and the dependence on oxygen represented by an application of nasal oxygen prongs, face mask, high-flow nasal oxygen (HFNO), non-invasive ventilation (NIV), intubation with invasive mechanical ventilation (IPPV), or the need for any form of the extracorporeal membrane oxygenation (ECMO)40. The available inflammatory markers (CRP, PCT, leucocyte/lymphocyte ratio) were recorded at start of therapy as well as plasmatic creatinine and the need for acute renal replacement therapy during hospital stay. The therapeutic factors potentially influencing the impact of remdesivir were concomitant administrations of monoclonal antibodies (Celltrion, RegnCov, Bamlanivimab, Evusheld), molnupiravir, and corticosteroids. These were recorded as either 6–8 mg of dexamethasone per day (e.g. low dose steroids), or more than 6–8 mg of dexamethasone or more than 40 mg of methylprednisolone per day (e.g. high dose steroids). Other factors with potential impact were coronavirus re-infection (i.e., previous test positivity), therapy with isoprinosine, tocilizumab, baricitinib, and any type of nationwide certified vaccination started at least 3 weeks before admission.
Anamnestic risk factors evaluated were hypertension, diabetes, ischaemic heart disease, asthma and COPD, chronic liver disease, haemato-oncologic history, history of immunosuppressive medication (corticosteroids, combined immunosuppressive therapy, biologic therapy), oncologic medical history incl. chemotherapy or radiotherapy in the last 6 months. The outcome data were ICU survival, hospital survival, and 1 year survival.
The endpoints of the study, namely the outcome of patients, as well as selected clinical records (e.g. vaccination status, time to ICU admission after vaccination, etc.) were controlled against population registries of the National Health Information System which is a database collecting all insurance data with 100% coverage of the Czech population.
The patients were included in two university hospitals in Prague. The General University Hospital is a prominent cardiac and ECMO centre, oncology centre, vasculitis centre, and it concentrates patients with multiple risk factors for the development of severe Covid-19. The University Hospital Kralovske Vinohrady is a complex trauma centre and intensive care department that covers a large area of eastern Prague. The ethical boards of both hospitals approved the study and confirm that all research was performed in accordance with the Declaration of Helsinki. A need for written informed consent was waived due to the retrospective nature of the study.
Statistical analysis
All analyses and data processing were performed in R 4.3.341 and RStudio 2023.12.141,42. Because most of the continuous data did not meet the criteria for normal distribution (Shapiro–Wilk test) they are expressed as median with 25th and 75th percentiles (interquartile range). Continuous parameters were compared by using the Wilcoxon test, the categorical parameters expressed as counts and percentages were compared using the chi-square or Fisher exact test. The primary and secondary outcomes were analysed using the chi-squared test, time to event analysis (Kaplan–Meier analysis with log rank test) and the univariate and multivariate Cox regression. The initial set of confounders for multivariate Cox regression (N = 19) was selected based on expert judgment. Subsequently, feature selection was performed using LASSO regularization with tenfold cross-validation using the glmnet 4.1-8 package43. For the multivariate model the missing values were input using the missForrest algorithm44. A p-value < 0.05 was considered statistically significant.
Results
Altogether, 339 patients (189 males, 55.8%), aged 71 (59; 77) years, were included in the analysis due to the detected presence of a viral load. The diagnosis was made by detecting NAg in the serum of 155 patients with a median of 217 (28; 1524) pg/ml, antigen detection in the sputum of 18 patients with a median of 18 (4.6; 32) COI, antigen detection in the nasopharyngeal samples of 44 patients with a median of 17 (8; 35) COI and the real-time PCR performed on nasopharyngeal samples of 122 patients with a median Ct of 21 (18; 27) (Fig. 1).
339 patients were selected according to the presence of viral load based on the positivity of the antigen tests or the real-time PCR. They were divided into the group without remdesivir therapy (199 patients, out of them 82 admitted to the ICU and 28 immune-supressed) and those treated with remdesivir (140 patients, out of them 103 admitted to the ICU and 57 immune-supressed).
140 of the 339 patients were treated with remdesivir, of those 103 in the ICU, and 57 of the 140 were treated with immune suppression. The median number of administered 100 mg remdesivir vials was 5.46 (1.08) per patient, which confirms a full 5-day treatment in the absolute majority of patients. Four patients (2.9%) of the remdesivir group presented as re-infections. The control group consisted of 199 patients who were denied therapy, of whom 82 were treated in the ICU and 28 of the 199 with immune suppression. Eight patients (4%) of the control group were re-infections.
Remdesivir patients were by 3 years younger (p = 0.005), more frequently with a history of immune suppression (40.7% vs. 14.1%, p < 0.001) and required more oxygen therapy (74.6% vs. 61.8%, p = 0.016, Table 1). Their inflammatory markers (CRP, PCT) and leucocyte counts were mildly higher (p < 0.001), their lymphocyte counts were lower resulting in a higher leucocyte-to-lymphocyte ratio (p < 0.001, Table 1). Regarding therapy, corticosteroids were administered more frequently in the remdesivir group (70% vs. 52.3%, p = 0.001), as well as monoclonal antibodies (39.3% vs. 8.5%, p < 0.001) and the immune stimulant isoprinosine (41.4% vs. 17.6%, p < 0.001). Patients on remdesivir were more frequently vaccinated more than 3 weeks prior to admission (42.9% vs. 11.6%, p < 0.001, Table 1). The recorded types of vaccines were Comirnaty (Pfizer, n = 69, 83%) or Vaxzevria (Astra-Zeneca, n = 14, 17%). Their hospital and long-term outcomes did not differ from the control group (Table 1).
The analysis of the ICU subgroup showed that patients treated with remdesivir had slightly higher morbidity scores (SOFA 8 (5;10) vs. 5 (3;9), p = 0.039, Table 2). They more often had a history of immune suppression (35.9% vs. 4.9%, p < 0.001), and their highest requirement for therapy of the respiratory insufficiency aligned more frequently with nasal oxygen prongs (13.6% vs. 1.2%, p = 0.002) or oxygen face mask (16.5% vs. 1.2%, p < 0.001). Their IPPV rates were similar to those of the control group, yet they were weaned more often with a tracheostomy than in the control group (42.5% vs. 22.4%, p = 0.01, Table 2). Their therapy frequently involved monoclonal antibodies against SARS-CoV-2 (39.8% vs. 7.3%, p < 0.001), isoprinosine (43.7% vs. 22%, p = 0.002) and prior vaccination (43.7% vs. 7.3%, p < 0.001, Table 2). Their ICU mortality (25.5%) was significantly lower than that of the control group (47.6%, p = 0.002), similarly the hospital mortality was lower in the remdesivir group (31.1% vs. 47.6%, p = 0.022, Table 2).
Analysis of the subgroup with immunosuppression showed that the remdesivir patients were by 7 years younger (p = 0.008), fewer had a history of oncology disease (29.8% vs. 85.7%, p < 0.001) and fewer were after radiotherapy (1.8% vs. 17.9%, p = 0.014, Table 3) than the control patients. In contrast, there were more haemato-oncology patients (45.6% vs. 14.3%, p = 0.005) and more patients on chronic corticosteroids (38.6% vs. 10.7%, p = 0.008) in the remdesivir treated group (Table 3). The immunosuppression group treated with remdesivir had higher inflammatory markers (CRP, leucocytes, p < 0.03) than the control group (Table 3) and a lower lymphocyte count (p = 0.008) associating with a significantly higher leucocyte-to-lymphocyte ratio (p = 0.01). Regarding therapy, patients on remdesivir were more frequently concomitantly treated with low dose steroids (68.4% vs. 35.7%, p = 0.004, Table 3) and monoclonal antibodies (49.1% vs. 3.6%, p < 0.001). They were also more often vaccinated than the controls (54.4% vs. 10.7%, p < 0.001). The hospital and 12-month outcomes of the immunosuppression patients treated with remdesivir did not differ from the controls (Table 3).
Regression analysis
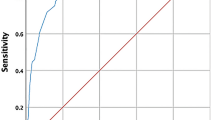
The univariate Cox regression (Table 4) demonstrated significant positive impacts on the adverse outcome of the male gender (HR 1.518, p = 0.027), admission to intensive care (HR 2.674, p < 0.001), intubation and IPPV (HR 2.966, p < 0.001). In contrast, maintaining patients with severe Covid-19 only with oxygen nasal prongs was associated with a better outcome (HR 0.49, p = 0.015) as well as the use of oxygen face mask (HR 0.548, p = 0.034). The multivariate adjusted Cox regression (LASSO) selected covariates significantly influencing outcomes (Table 4). These were remdesivir (HR 0.66, p = 0.039, Fig. 2), admission to the ICU (HR 2.43, p = 0.006) and intubation with IPPV (HR 3.04, p < 0.001). Nine missing CRP values, ten leukocyte/lymphocyte ratio values and four creatinine values were imputed using the missForrest algorithm. The other most significant confounders were age, ICU admission, mechanical ventilation, leucocyte/lymphocyte ratio and admission creatinine.
Univariate Cox regression (A) and the multivariate adjusted Cox regression (LASSO) (B) comparing 12-month survival of all the patients with remdesivir to controls. The univariate 12-month survival analysis (C), and multivariate adjusted Cox regression (LASSO) (D) for remdesivir treated subset of ICU patients versus controls.
For the subset of 185 ICU patients the univariate Cox regression (Table 5) demonstrated significant impacts on the adverse outcome of hypertension history (HR 1.65, p = 0.029), history of immune suppression (HR 1.62, p = 0.038), intubation and IPPV (HR 2.15, p = 0.004). In contrast, maintaining patients with severe Covid-19 on nasal oxygen prongs improved the outcome (HR 0.29, p = 0.037) as well as the use of oxygen face mask (HR 0.24, p = 0.016). The remdesivir therapy improved the outcome (HR 0.65, p = 0.043). The multivariate adjusted Cox regression (LASSO) selected covariates significantly influencing outcomes (Table 5). These were remdesivir (HR for adverse outcome 0.49, p = 0.006, Fig. 2) and intubation with IPPV (HR 2.55, p = 0.001). Eight missing CRP values, eight leukocyte/lymphocyte ratio values and two creatinine values were imputed using the missForrest algorithm. The other most significant confounders were age, leucocyte/lymphocyte ratio, admission creatinine and immunosuppression.
For the subset of 85 patients with immune suppression the univariate Cox regression (Table 6) demonstrated significant impacts on the adverse outcome of the male gender (HR 2.31, p = 0.017), history of COPD (HR 2.37, p = 0.021), ICU admission (HR 4.48, p < 0.001), intubation and IPPV (HR 3.03, p = 0.001). The multivariate adjusted Cox regression (LASSO) selected covariates significantly influencing outcomes (Table 6). These were a history of COPD (HR for adverse outcome 2.81, p = 0.012) and ICU admission (HR 6.01, p = 0.005). The impact of remdesivir did not reach statistical significance. One leukocyte to lymphocyte ratio value was imputed using the missForrest algorithm.
Discussion
Although remdesivir was administered more frequently to patients with immune suppression including with haemato-oncology diseases who required more oxygen therapy than the controls, were more lymphopenic and had a more unfavourable course of the inflammatory markers, the drug has proven its benefits in patients with a high viral load. The impact on short and long-term outcomes was remarkable especially in patients with higher illness severity, e.g., in the intensive care unit, which contrasts with the guidelines for the administration of remdesivir applied throughout the pandemic.
Interestingly, patients on remdesivir were more often managed without mechanical ventilation, e.g., on nasal oxygen cannulas or face masks, which could have brought an associated prognostic benefit in comparison to intubation and mechanical ventilation which is in line with published data1,7,40,45. The adverse prognostic impact of an ICU admission and mechanical ventilation has been confirmed particularly in the immune compromised patients37,39. This study confirmed unfavourable impacts of the male gender, history of hypertension, COPD, or immune suppression on the short and long-term outcomes of severe Covid-19. Of the therapeutic factors, the group with remdesivir therapy was more frequently vaccinated (e.g., more than 3 weeks after the first jab), and this did not transmit in any favourable outcome parameter. The associated illness severity and the high proportion of patients with immune suppression with a poor previous immune response to vaccination could help to explain its limited protective effects on patient outcomes46,47. Similarly, the application of the monoclonal antibodies against the coronavirus spike protein, which was more frequent in the remdesivir group, was not associated with any outcome data. These results allude to a high illness severity of the intensive care patients which was likely less in the Recovery trial admitting a benefit of the monoclonal antibodies even for patients admitted to the hospital with Covid-1948. The immunity stimulant isoprinosine found more often in the remdesivir group has not shown any significant impact on the outcomes in the multivariate regression analysis. The number of re-infections among the patients on remdesivir and in the control group were negligible.
Although previous, mostly retrospective studies reported a benefit of remdesivir in slowing the progression of severe Covid19 and avoidance of admission to the ICU, mechanical ventilation, and death, this research suggests a benefit specifically for patients in the ICU with very severe disease and detected load of a living virus. In general, the viral load is a key to understanding the illness severity and the immune response. A high load of living virus has been correlated with the probability of intubation, mechanical ventilation, and death45,49,50,51. It is also associated with an immune response, seroconversion, and the native immunisation with coronavirus of the general population29,52.
This has a substantial implication for adequate testing. A qualitative PCR from a nasopharyngeal or sputum sample may represent only mucosal infection or colonisation that has been associated with approximately 50% chance for triggering of a systemic immune response, thus resulting in a similar probability of seroconversion and a build-up of the native immunity which is associated with outcomes52,53. In other words, patients with only qualitative PCR mucosal positivity without considering any clinical symptoms may have about 50% chance for re-infection due to the absence of systemic immune response52. Therefore, assessing the viral load with some of the available tests would be crucial for the prognosis and for the indication of antiviral medication which would be more targeted and justified than in subjects tested only by means of a qualitative PCR.
In 64% of our selected cohort of 339 patients, the viral load was detected by suitable antigen tests performed in certified laboratory settings. Since early 2021 the antigen tests have been tested as accurate not only for symptomatic patients but also for oligosymptomatic and asymptomatic subjects with possible implications for population screening27,47,54. The specificity of antigen tests for detecting the presence of a viral load makes them a great tool for the detection of high-risk patients and the target population for the antiviral therapy. The requirements for antigen tests have been published27 and their growing popularity has also been supported by financial savings compared to a real-time PCR. The economic aspect of our study is not only related to testing, but also to the costly administration of remdesivir, which may become more medically and economically efficient when targeting patients with a viral load detected by modern antigen tests55,56,57.
The limitations of the study are multiple. This is a retrospective study on selected severe Covid-19 patients with a detected living virus—in contrast to other studies working with only qualitative PCR positivity which, we believe, may not be sufficient for the administration of the medication against the coronavirus. Our diagnosis of viral load are only estimates based on the certified laboratory tests and not controlled by a qualitative viral analysis. By selecting a rather large group that tested positive for viral load, we cannot exclude the effects of remdesivir on another subset of patients, for example, less burdened with an immune compromise and a cumulation of risk factors. Specifically, for patients with immune compromise, the study was likely underpowered, and we cannot exclude the benefits of remdesivir with respect to the reported prolonged replication of the coronavirus in these patients10,11,28,37. There is also a potential problem with the inclusion of all oncology treated patients, those on immune suppressants and chronic dialysis into one group as these diseases are heterogenous in their response to coronavirus, immune interventions, and a vaccination35,36,46. The median remdesivir dosage represents a full five-day therapy in the absolute majority of patients included, we cannot dismiss an impact of a shorter therapy indicated in less severe patients with a presence of a viral load. For comparison, we show the differences between the univariate and the multivariate regression analysis. Regardless of the LASSO multivariate Cox analysis we cannot fully exclude a bias given by the complex therapeutic interventions to severe Covid-19 patients, especially in the ICU.
Conclusions
Despite advanced Covid-19 and high illness severity of the intensive care patients the therapy with remdesivir associated with the outcome benefits in patients with a presence of viral load, which was estimated by widely available antigen tests in majority of patients or less frequently by the real-time PCR with determination of the cycle threshold. The result may serve as a basis for further prospective research to better justify antiviral therapy in the advanced stages of the disease.
Data availability
The anonymous study dataset is available upon reasonable request to the authors (email of the corresponding author martin.balik@vfn.cz).
References
Amstutz, A. et al. Effects of remdesivir in patients hospitalised with COVID-19: A systematic review and individual patient data meta-analysis of randomised controlled trials. Lancet Respir. Med. 11, 453–464 (2023).
Beigel, J. H. et al. Remdesivir for the treatment of Covid-19 - final report. N. Engl. J. Med. 383, 1813–1826 (2020).
Frediansyah, A., Nainu, F., Dhama, K., Mudatsir, M. & Harapan, H. Remdesivir and its antiviral activity against COVID-19: A systematic review. Clin. Epidemiol. Glob. Health 9, 123–127 (2021).
Gottlieb, R. L. et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N. Engl. J. Med. 386, 305–315 (2022).
Spinner, C. D. et al. Effect of remdesivir versus standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. Jama 324, 1048–1057 (2020).
Alonso-Navarro, R. et al. Time from symptoms onset to remdesivir is associated with the risk of ICU admission: A multicentric analyses. BMC Infect. Dis. 23, 286 (2023).
Ader, F. et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomised, controlled, open-label trial. Lancet Infect. Dis. 22, 209–221 (2022).
Akiyama, S., Hamdeh, S., Micic, D. & Sakuraba, A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: A systematic review and meta-analysis. Ann. Rheum. Dis. 80, 384–391 (2021).
Lee, L. Y. W. et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 21, 1309–1316 (2020).
Pablos, J. L. et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: A multicentric matched cohort study. Ann. Rheum. Dis. 79, 1544–1549 (2020).
Passamonti, F. et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: A retrospective, multicentre, cohort study. Lancet Haematol. 7, e737–e745 (2020).
Ali, K. et al. Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: A randomized controlled trial. Cmaj 194, E242-e251 (2022).
Garibaldi, B. T. et al. Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients With COVID-19. JAMA Netw. Open 4, e213071 (2021).
Grasselli, G. et al. Risk factors associated with mortality among patients With COVID-19 in intensive care units in Lombardy Italy. JAMA Intern. Med. 180, 1345–1355 (2020).
Pan, H. et al. Remdesivir and three other drugs for hospitalised patients with COVID-19: Final results of the WHO Solidarity randomised trial and updated meta-analyses. Lancet 399, 1941–1953 (2022).
Adamsick, M. L. et al. Remdesivir in patients with acute or chronic kidney disease and COVID-19. J. Am. Soc. Nephrol. 31, 1384–1386 (2020).
Aiswarya, D. et al. Use of remdesivir in patients With COVID-19 on hemodialysis: A study of safety and tolerance. Kidney Int. Rep. 6, 586–593 (2021).
Cillóniz, C. et al. Remdesivir and survival outcomes in critically ill patients with COVID-19: A multicentre observational cohort study. J. Infect. 86, 256–308 (2023).
Goldberg, E. et al. A real-life setting evaluation of the effect of remdesivir on viral load in COVID-19 patients admitted to a large tertiary centre in Israel. Clin. Microbiol. Infect. 27(917), e911-917.e914 (2021).
Marrone, A. et al. Remdesivir plus dexamethasone versus dexamethasone alone for the treatment of coronavirus disease 2019 (COVID-19) patients requiring supplemental O2 therapy: A prospective controlled nonrandomized study. Clin. Infect. Dis. 75, e403–e409 (2022).
Bauer, R. N. et al. Prognostic value of severe acute respiratory syndrome coronavirus-2 viral load and antibodies in patients hospitalized with COVID-19. Clin. Transl. Sci. 16, 1049–1062 (2023).
Bergwerk, M. et al. Covid-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 385, 1474–1484 (2021).
Lingas, G. et al. Effect of remdesivir on viral dynamics in COVID-19 hospitalized patients: A modelling analysis of the randomized, controlled, open-label DisCoVeRy trial. J. Antimicrob. Chemother. 77, 1404–1412 (2022).
Bermejo-Martin, J. F. et al. Viral RNA load in plasma is associated with critical illness and a dysregulated host response in COVID-19. Crit. Care 24, 691 (2020).
Jittamala, P. et al. Clinical antiviral efficacy of remdesivir in coronavirus disease 2019: An open-label, randomized controlled adaptive platform trial (PLATCOV). J. Infect. Dis. 228, 1318–1325 (2023).
Pujadas, E. et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir. Med. 8, e70 (2020).
Drain, P. K. Rapid diagnostic testing for SARS-CoV-2. N. Engl. J. Med. 386, 264–272 (2022).
Singanayagam, A. et al. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill. 25, 2001483 (2020).
Marks, M. et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: A cohort study. Lancet Infect. Dis. 21, 629–636 (2021).
La Scola, B. et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur. J. Clin. Microbiol. Infect. Dis. 39, 1059–1061 (2020).
Corman, V. M. et al. Comparison of seven commercial SARS-CoV-2 rapid point-of-care antigen tests: A single-centre laboratory evaluation study. Lancet Microbe 2, e311–e319 (2021).
Corey, L. et al. SARS-CoV-2 variants in patients with immunosuppression. N. Engl. J. Med. 385, 562–566 (2021).
Frampton, D. et al. Genomic characteristics and clinical effect of the emergent SARS-CoV-2 B.1.1.7 lineage in London, UK: A whole-genome sequencing and hospital-based cohort study. Lancet Infect. Dis. 21, 1246–1256 (2021).
Ong, S. W. X. et al. Clinical and virological features of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants of concern: A retrospective cohort study comparing B.1.1.7 (Alpha), B.1.351 (Beta), and B.1.617.2 (Delta). Clin. Infect. Dis. 75, e1128–e1136 (2022).
Benotmane, I. et al. In-depth virological assessment of kidney transplant recipients with COVID-19. Am. J. Transplant. 20, 3162–3172 (2020).
Benotmane, I., Risch, S., Doderer-Lang, C., Caillard, S. & Fafi-Kremer, S. Long-term shedding of viable SARS-CoV-2 in kidney transplant recipients with COVID-19. Am. J. Transplant. 21, 2871–2875 (2021).
Lahmer, T. et al. SARS-CoV-2 viral load dynamics in immunocompromised critically ill patients on remdesivir treatment. Multidiscip. Respir. Med. 17, 825 (2022).
Razzack, A. A. et al. A meta-analysis of association between remdesivir and mortality among critically-Ill COVID-19 patients. Infect. Chemother. 53, 512–518 (2021).
Ryoo, S. et al. The effects of remdesivir on mortality and the requirement for mechanical ventilation in patients with COVID-19: A systematic review stratified by disease severity. Korean J. Intern. Med. 39, 160–171 (2024).
Marshall, J. C. et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect. Dis. 20, e192–e197 (2020).
Team, R.C. A language and environment for statistical computing in https://www.R-project.org (ed. Computing, R.F.f.S.) (Vienna, 2022).
team, P. RStudio: integrated development environment for R. Posit Software. in http://www.posit.co, Vol. 2022 (ed. PBC) (Boston, MA, 2022).
Simon, N., Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for Cox’s proportional hazards model via coordinate descent. J. Stat. Softw. 39, 1–13 (2011).
Stekhoven, D. J. & Bühlmann, P. MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics 28, 112–118 (2012).
Magleby, R. et al. Impact of severe acute respiratory syndrome coronavirus 2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019. Clin. Infect. Dis. 73, e4197–e4205 (2021).
Mair, M. J. et al. Humoral immune response in hematooncological patients and health care workers who received SARS-CoV-2 vaccinations. JAMA Oncol. 8, 106–113 (2022).
Stephens, D. S. & McElrath, M. J. COVID-19 and the path to immunity. Jama 324, 1279–1281 (2020).
RECOVERY Collaborative Group. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 399, 665–676 (2022).
Fourati, S., Hue, S., Pawlotsky, J. M., Mekontso-Dessap, A. & de Prost, N. SARS-CoV-2 viral loads and serum IgA/IgG immune responses in critically ill COVID-19 patients. Intensiv. Care Med. 46, 1781–1783 (2020).
Rogers, A. J. et al. The association of baseline plasma SARS-CoV-2 nucleocapsid antigen level and outcomes in patients hospitalized With COVID-19. Ann. Intern. Med. 175, 1401–1410 (2022).
Westblade, L. F. et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell 38, 661-671.e662 (2020).
Ma, Q. et al. Global percentage of asymptomatic SARS-CoV-2 infections among the tested population and individuals with confirmed COVID-19 diagnosis: A systematic review and meta-analysis. JAMA Netw. Open 4, e2137257 (2021).
Liu, W. et al. Predictors of nonseroconversion after SARS-CoV-2 infection. Emerg. Infect. Dis. 27, 2454–2458 (2021).
Drain, P. K. et al. Accuracy of 2 rapid antigen tests during 3 phases of SARS-CoV-2 variants. JAMA Netw. Open 5, e2228143 (2022).
Congly, S. E., Varughese, R. A., Brown, C. E., Clement, F. M. & Saxinger, L. Treatment of moderate to severe respiratory COVID-19: A cost-utility analysis. Sci. Rep. 11, 17787 (2021).
Lau, V. I. et al. Cost-effectiveness of remdesivir plus usual care versus usual care alone for hospitalized patients with COVID-19: an economic evaluation as part of the Canadian Treatments for COVID-19 (CATCO) randomized clinical trial. CMAJ Open 10, E807-e817 (2022).
Wong, C. K. H. et al. Clinical improvement, outcomes, antiviral activity, and costs associated with early treatment with remdesivir for patients with coronavirus disease 2019 (COVID-19). Clin. Infect. Dis. 74, 1450–1458 (2022).
Acknowledgements
The authors thank Dr. M.C.Mokotedi for proofreading and English editing.
Funding
The protocol has received a two-year (2022–2024) grant support from the Czech Health Research Council, AZV NU22-B-147, commencing on the 1st of April 2022.
Author information
Authors and Affiliations
Contributions
B.M.: design, conceptualization, funding, methodology, statistics, writing; W.P.: design, methodology, electronic case report form, statistics, writing; S.E.: data collection, writing; J.I.: data collection; D.M.: data collection; Z.J.: methodology, data collection; S.G.: methodology, data collection; K.L.: methodology; A.V.: methodology, writing; F.M.: data collection;, J.K.: data collection; M.J.: data collection; T.T.: data collection, writing; D.F.: design, methodology; D.L.: methodology, data extraction from the National Health Information System.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Balik, M., Waldauf, P., Jurisinova, I. et al. SARS-CoV-2 viral load is linked to remdesivir efficacy in severe Covid-19 admitted to intensive care. Sci Rep 14, 20825 (2024). https://doi.org/10.1038/s41598-024-71588-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71588-9