Abstract
Patients with gastric cancer (GC) are more susceptible to coronavirus disease 2019 (COVID-19), which further worsens their already challenging prognosis. However, there are no effective treatment options for these patients. Lentinan is a potent bioactive component with antiviral and antitumor effects. We hypothesized that lentinan might exert powerful pharmacological effects in patients with GC and COVID-19. In this study, a prognostic model of patients with GC/COVID-19 was constructed and used to apply a network pharmacology approach to reveal biological functions, drug targets, and molecular mechanisms of the action of lentinan against GC/COVID-19. Clinical analysis revealed key prognostic genes in patients with GC/COVID-19. The results of network pharmacology analysis suggested that the therapeutic effect of lentinan on GC/COVID-19 mainly involves the modulation of several neutrophil-related biological processes, as well as the nucleotide-binding and oligomerization domain-like receptor and interleukin-17 signaling pathways. In addition, C-X-C motif chemokine ligand 8, vascular endothelial growth factor A, ribonuclease 3, and F2 were identified as key genes of lentinan against GC/COVID-19. Key prognostic genes were identified in patients with GC/COVID-19 through the construction of a prognostic model. Pharmacological functions and signaling pathways of lentinan against GC/COVID-19 were revealed. These included the regulation of neutrophils and NOD-like receptor signaling pathways. The findings provide the first published evidence of the potential value of lentinan as a complementary therapy for GC/COVID-19.
Similar content being viewed by others
Introduction
As of June 2024, there have been more than 775 million confirmed cases of coronavirus disease 2019 (COVID-19) worldwide and more than 7 million deaths (Covid19.who.int/). Currently, there are no effective treatment for COVID-19. In addition, it has been reported that the risk of COVID-19 infection among hospitalized cancer patients is 2.31 times higher than that of the general population1. Gastric cancer (GC) is the fifth most prevalent cancer and the third leading cause of cancer-related deaths, with over one million cases diagnosed annually2. In the majority of the world, GC has a mortality rate of 75% 3. The treatment of GC patients who acquire COVID-19 is often complicated by their poor nutritional status and immune response4. Effective therapeutic options for such patients are needed.
Lentinus edodes (dubbed “the king of mushrooms”) is a medicinal and edible fungus. Its main active substance is lentinan5. Lentinan is a macromolecule with a main chain of β-1,3-D-glucopyranose that branches every five glucose units consisting of two β-1,6-bonded glucopyranose residues6. Lentinan can induce antitumor immune responses that involved activated macrophages, T lymphocytes, B lymphocytes, natural killer cells, and other immune cells7. Lentinan also has anticancer activity, which involves controlling caspase-3 expression8, telomerase activity9, and cytochrome P450 (CYP) production10. Moreover, lentinan exerts pronounced antiviral activity against infectious hematopoietic cell necrosis virus11, herpes simplex virus type 1 12, and human immunodeficiency virus13. However, the activity of lentinan against COVID-19 and whether can be used to treat patients with both GC and COVID-19 (GC/COVID-19) are unclear.
Network pharmacology14,15 is an efficient identification technique for key targets, molecular functions, and biological processes involved in the treatment of clinical diseases with bioactive compounds. In this study, we investigated the potential mechanism of lentinan intervention in GC/COVID-19 using a network pharmacology approach. The findings reveal a potentially valuable complementary treatment option for GC/COVID-19 patients (Fig. 1).
Materials and methods
Identification of GC/COVID-19–associated genes
To identify genes associated with GC/COVID-19, transcriptomic data from TCGA (https://portal.gdc.cancer.gov/) was downloaded, comprising 375 tumor samples and 32 normal tissue samples. As well, data of the clinical characteristic for 443 GC patients was obtained16. The “limma” package in R (3.6.3) was used to screen and obtain DEGs from GC patients with a false discovery rate < 0.05 and |logfold change (FC)| > 1. Genes associated with COVID-19 were obtained from the Genecard, OMIM, and NCBI gene databases17. The resulting GC/COVID-19 genes were compared, and the intersection was considered.
Development and validation of a GC/COVID-19 prognostic model
A Perl script was used to integrate the GC/COVID-19-related genes with the survival time and survival status data of GC patients. The “survival” package of R (3.6.3) was used to perform univariate and multifactor Cox analyses on the integrated data18. Kaplan–Meier survival curves were used to verify the relationship between risk class and overall survival of patients. ROC curves were used to assess the accuracy of prognostic models. Independent prognostic analyses of clinical factors and risk scores were performed on univariate and multifactorial bases. Finally, the constructed prognostic model was validated in each clinical subgroup.
Determination of lentinan-pharmacological targets in GC/COVID-19
We obtained the target genes of lentinan from the Herb19, Swiss Target Prediction20 and PharmMapper21 databases. The targets obtained from the latter database were annotated using UniProt. The targets obtained from the three databases were compared with GC/COVID-19 related genes, and intersections were considered.
Enrichment analyses
GO and KEGG22,23,24 enrichment of lentinan/GC/COVID-19-associated genes were performed using “org.Hs.eg.db” and “clusterProfiler” packages of R (3.6.3)25. GO terms with p < 0.05 were considered statistically significant. Associations between lentinan/GC/COVID-19-related genes, GO terms, and KEGG pathways were visualized using Cytoscape 3.9.0 (https://cytoscape.org/) .
PPI network map
All lentinan/GC/COVID-19-related genes were entered into the STRING database (https://string-db.org/). The combined score was set at > 0.4 to obtain the protein network interactions of the intersecting genes. The results were imported into Cytoscape 3.9.0 for visualization to construct the PPI network.
Molecular docking
Molecular docking can be used to predict the binding and interactions between proteins and small molecules. The crystal structure of the target protein was downloaded from the PDB database. PyMol 2.5 (https://pymol.org/) was used to delete the water molecules and ligands in the crystal structure. AutoDockTools (1.5.6) was used for hydroprocessing. The final results were saved as a pdbqt file. The SDF format file of the two-dimensional structure of lentinan was obtained from the PubChem database, converted to the three-dimensional structure using Chem3D (14.0), and energy-minimized by molecular dynamics calculations using the MM2 method exported as a mol2 format file. AutoDockTools (1.5.6) was used to process the mol2 file. AutoDock vina26 was used for molecular docking. Each docking generated a total of nine conformations. The conformation with the highest affinity was chosen as the final docking conformation and was represented in PyMoL 2.5.
Results
Identification of GC/COVID-19-associated genes
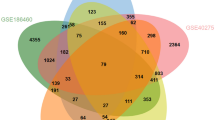
We compiled 4672 COVID-19-associated genes from the Online Mendelian Inheritance in Man (OMIM), National Center for Biotechnology Information (NCBI), and Genecard databases (Fig. 2A). The Cancer Genome Atlas (TCGA) database was screened for 8893 differentially expressed genes (DEGs) between tumor and normal tissues of GC patients. Aligning these two gene sets revealed 683 overlapping genes between GC and COVID-19 (Fig. 2A). Of the 683 overlapping genes, 552 genes were upregulated, and 131 genes were downregulated in tumor tissues of GC patients (Fig. 2B).
Development and validation of GC/COVID-19 prognostic model
To determine the relationship between GC/COVID-19-related genes and the clinicopathological features of patients with GC/COVID-19, univariate and multifactorial Cox analyses were performed on the 683 overlapping genes. Univariate Cox analysis identified 19 genes that were significantly associated with GC/COVID-19 (P < 0.05; Table 1). Of these genes, multivariate Cox analysis revealed a significant association of GC/COVID-19 and five genes (F5, SERPINE1, F2, RNASE3, and KYNU; Table 2). Based on the coefficient values of the multivariate Cox analysis, patients were divided into a high-risk and low-risk group (Table 2; Fig. 3A). Patients with higher risk values had lower survival rates (Fig. 3B) and were associated with elevated expression levels of F5, SERPINE1, F2, RNASE3, and KYNU (Fig. 3C). Survival analysis revealed that the overall survival rate of patients in the low-risk group was higher than that of the patients in the high-risk group (Fig. 3D). The prognostic model was highly accurate in predicting 5-year survival, based on area under the receiver operation characteristic (ROC) curve (AUC > 0.7; Fig. 3E). Independent prognostic univariate (Fig. 4A) and multifactorial (Fig. 4B) analyses revealed the risk score as an independent risk factor affecting patient prognosis (Table 3). The patients were subgrouped according to age, sex, grade, and stage. The prognostic model was validated among the different subgroups. Survival of patients in the low-risk group was significantly longer than that of patients in the high-risk group in the different subgroups, except for the distant tumor metastasis (M1) subgroup (Fig. 5A-N). The negative results in the M1 subgroup may have been caused by the low number of cases.
Prognostic value of GC/COVID-19- associated genes. (A) Risk scores for patients in the high- and low-risk groups. (B) Survival of patients in the high- and low-risk groups. (C) Heat map of the expression of F5, SERPINE1, F2, RNASE3, and KYNU in tumor tissues of patients in high- and low-risk groups. (D) Survival analysis of patients in the high- and low-risk groups. (E) Receiver operating characteristic (ROC) curve of the prognostic model.
Identification of core targets of lentinan against GC/COVID-19
We obtained 240 lentinan target genes from the Herb, Swiss Target Prediction, and PharmMapper databases. A comparison of the GC/COVID-19-related genes with the target genes of lentinan identified 22 overlapping genes (Fig. 6A). Gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed on these 22 genes. The analyses revealed the involvement of lentinan in the regulation of a series of biological processes that included neutrophil activation (including activation involved in the immune response), neutrophil-mediated immunity, neutrophil degranulation, collagen metabolic process, leukocyte migration, collagen catabolic process, regulation of inflammatory response, covalent chromatin modification, and response to oxidative stress (Figs. 6B and 7). Ten KEGG pathways related to all core targets were found. They included the nucleotide-binding and oligomerization domain (NOD)-like receptor signaling pathway, prostate cancer, interleukin-17 (IL-17) signaling pathway, rheumatoid arthritis, fluid shear stress and atherosclerosis, measles, and Th17 cell differentiation (Figs. 6C and 7).
Molecular docking of lentinan with potential core targets
The STRING database was used to obtain the interactions between the lentinan/GC/COVID-19- associated genes. Cytoscape software was used to visualize and construct the protein–protein interaction (PPI) network (Fig. 8). Based on the degree value of the PPI network, we identified C-X-C motif chemokine ligand 8 (CXCL8) and vascular endothelial growth factor A (VEGFA) as the core targets of lentinan against GC/COVID-19. In addition, F2 and ribonuclease 3 (RNASE3) were identified as lentinan anti-GC/COVID-19 related genes and key prognostic genes in GC/COVID-19 patients. To determine the possible binding of lentinan to proteins encoded by CXCL8, VEGFA, F2, and RNASE3, a molecular docking analysis was performed. The molecular structures of proteins encoded by CXCL8, VEGFA, F2, and RNASE3 were obtained from the PDB database (6n2u, 6zfl, 6e09, and 4 × 08, respectively). Lower binding energy of the ligand to the receptor indicated a tighter binding conformation between them. The lowest binding energies of lentinan to proteins encoded by CXCL8, VEGFA, F2, and RNASE3 were − 5.9, -7.6, -8.4, and − 10.3 kcal/mol, respectively. Lentinan may have a strong binding effect on these proteins (Table 4; Fig. 9).
Discussion
GC patients have an older average age, more complications, repeated hospital visits due to treatment needs, and are often immunocompromised due to antitumor therapy. If they become infected with severe acute respiratory syndrome coronavirus 1, which causes COVID-19, the disease will progress faster and can be more severe, which increases mortality27,28,29,30. In this study, we screened the DEGs in GC patients and aligned them with COVID-19-related genes to obtain 683 intersecting genes. Among these genes, 552 were upregulated and 131 genes were downregulated in the tumor tissues of GC/COVID-19 patients. Based on independent prognostic and survival analyses, a few important DEGs, including F5, SERPINE1, F2, RNASE3, and KYNU, were implicated as effective biomarkers for the screening and identification of GC/COVID-19 patients at different risk stages.
Lentinan has significant antitumor effects against GC31. Given the antiviral activity of lentinan32, we speculated that it may have a powerful pharmacological effect in GC/COVID-19 patients. Using network pharmacology, we further screened 22 intersecting genes of lentinan/GC/COVID-19. GO enrichment analysis showed that in GC/COVID-19, lentinan mainly regulates several neutrophil-related biological processes, including neutrophil activation (including the activation involved in immunity), neutrophil-mediated immunity, neutrophil degranulation, among others. The most enriched KEGG pathways were the NOD-like receptor signaling pathway, prostate cancer, IL-17 signaling pathway, rheumatoid arthritis, and others. NOD-like receptors are important pattern recognition receptors in innate immune responses33, which can directly participate in the signal transduction of viral invasion34,35 and affect tumor progression by regulating tumor necrosis factor, Toll-like receptor, and other signaling pathways36,37. IL-17, the main effector of Th17 cells38, induces a systemic increase in the leukocyte chemokine granulocyte-colony stimulating factor, leading to massive neutrophil infiltration around the tumor, inhibition of CD8 + cytotoxic T lymphocytes, and promotion of tumor cell metastasis39. Notably, COVID-19 patients also have increased neutrophils in their blood and lungs40. Activated neutrophils induce the production of reactive oxygen species and cause lung tissue damage41,42. Dysregulation of neutrophil elastase also disrupts the alveolar–capillary barrier43,44. Additionally, viral infection can induce the release of neutrophil extracellular traps45. Elevated plasma levels of neutrophil extracellular traps in COVID-19 patients reportedly lead to lung injury and microvascular thrombosis, which can increase the severity of COVID-19 46. The increase and activation of neutrophils have been regarded as an indicator of poor prognosis in COVID-19 patients47. The results of the enrichment analysis showed that the biological processes and signaling pathways involved in the 22 genes related to lentinan/GC/COVID-19 were strongly correlated with GC/COVID-19. The findings implicate these 22 genes as effective targets of lentinan in the treatment of GC/COVID-19.
Analysis of the degree values of the PPI network revealed that the main targets of lentinan intervention in GC/COVID-19 were IL-8 (CXCL8) and VEGFA. IL-8 is involved in the IL-17 signaling pathway (Supplementary Table 2) and has a strong catalytic effect on neutrophils48. VEGFA is a key cytokine that regulates tumor angiogenesis49. RNASE3 and F2 were also lentinan anti-GC/COVID-19 related genes and key prognostic genes in GC/COVID-19 patients. RNASE3 is a member of the RNase A superfamily involved in host immunity. RNASE3 regulation of macrophages has an anti-infection role50. F2 encodes prothrombin. Coagulation disorders are frequently in critically ill COVID-19 patients and have become a useful way to differentiate the severity of COVID-19 51. Through molecular docking analysis, we demonstrated the stable binding of lentinan to proteins encoded by CXCL8, VEGFA, RNASE3, and F2.
The collective findings suggest that lentinan may be an effective agent for treating patients with GC/COVID-19.
Conclusion
In this study, key prognostic genes were identified in patients with GC/COVID-19 through the construction of a prognostic model. Pharmacological functions and signaling pathways of lentinan against GC/COVID-19 were revealed. These included the regulation of neutrophils and NOD-like receptor signaling pathways. The findings provide the first published evidence of the potential value of lentinan as a complementary therapy for GC/COVID-19. Further clinical experiments are required to verify the conclusions of this study.
Data availability
The data used to support the findings of this study are available within the article and supplementary tables.
References
Yu, J., Ouyang, W., Chua, M. L. K. & Xie, C. SARS-CoV-2 transmission in patients with Cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol. 6 (7), 1108–1110 (2020).
Thrift, A. P. & El-Serag, H. B. Burden of gastric Cancer. Clin. Gastroenterol. Hepatol. 18 (3), 534–542 (2020).
Karimi, P., Islami, F., Anandasabapathy, S., Freedman, N. D. & Kamangar, F. Gastric Cancer: descriptive epidemiology, risk factors, screening, and Prevention. Cancer Epidemiol. Biomarkers Prev. 23 (5), 700–713 (2014).
Makeeva, Т. K. & Galkin, A. A. Nutritional status of the gastric cancer patients. Vestnik Saint Petersburg Univ. Med. S1, 105–117 (2008).
Chihara, G., Maeda, Y., Hamuro, J., Sasaki, T. & Fukuoka, F. Inhibition of mouse sarcoma 180 by polysaccharides from Lentinus edodes (Berk.) Sing. Nature. 222 (5194), 687–688 (1969).
Zhang, Y. Y., Li, S., Wang, X. H., Zhang, L. N. & Cheung, P. C. K. Advances in lentinan: isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 25 (2), 196–206 (2011).
Ren, L., Perera, C. & Hemar, Y. Antitumor activity of mushroom polysaccharides: a review. Food Funct. 3 (11), 1118–1130 (2012).
Lull, C., Wichers, H. J. & Savelkoul, H. F. Antiinflammatory and immunomodulating properties of fungal metabolites. Mediators Inflamm. 2005 (2), 63–80 (2005).
Sreenivasulu, K., Vijayalakshmi, M. & Sambasivarao, K. R. Regulation studies of telomerase gene in cancer cells by lentinan. Avicenna J. Med. Biotechnol. 2 (4), 181–185 (2010).
Hashimoto, T. et al. Suppressive effect of polysaccharides from the edible and medicinal mushrooms, Lentinus edodes and Agaricus Blazei, on the expression of cytochrome P450s in mice. Biosci. Biotechnol. Biochem. 66 (7), 1610–1614 (2002).
Ren, G. M., Xu, L. M., Lu, T. Y. & Yin, J. S. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int. J. Biol. Macromol. 115, 1202–1210 (2018).
Sarkar, S., Koga, J., Whitley, R. J. & Chatterjee, S. Antiviral effect of the extract of culture medium of Lentinus edodes mycelia on the replication of herpes simplex virus type 1. Antiviral Res. 20 (4), 293–303 (1993).
Suzuki, H. et al. Inhibition of the infectivity and cytopathic effect of human immunodeficiency virus by water-soluble lignin in an extract of the culture medium of Lentinus edodes mycelia (LEM). Biochem. Biophys. Res. Commun. 160 (1), 367–373 (1989).
Wang, X., Wang, Z. Y., Zheng, J. H. & Li, S. TCM network pharmacology: a new trend towards combining computational, experimental and clinical approaches. Chin. J. Nat. Med. 19 (1), 1–11 (2021).
Luo, T. T. et al. Network Pharmacology in Research of Chinese Medicine Formula: methodology, application and prospective. Chin. J. Integr. Med. 26 (1), 72–80 (2020).
Liu, J. et al. An Integrated TCGA Pan-cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell. 173 (2), 400– (2018).
Li, R. et al. Network Pharmacology and bioinformatics analyses identify intersection genes of niacin and COVID-19 as potential therapeutic targets. Brief. Bioinform. 22 (2), 1279–1290 (2021).
Fisher, L. D. & Lin, D. Y. Time-dependent covariates in the Cox proportional-hazards regression model. Annu. Rev. Public. Health. 20, 145–157 (1999).
Fang, S. et al. HERB: a high-throughput experiment- and reference-guided database of traditional Chinese medicine. Nucleic Acids Res. 49 (D1), D1197–D1206 (2021).
Daina, A., Michielin, O. & Zoete, V. Swiss target prediction: updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 47 (W1), W357–W364 (2019).
Wang, X. et al. PharmMapper 2017 update: a web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 45 (W1), W356–W360 (2017).
Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28 (1), 27–30 (2000).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28 (11), 1947–1951 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51 (D1), D587–d592 (2023).
Yu, G., Wang, L-G., Han, Y. & He, Q-Y. clusterProfiler: an R Package for comparing Biological themes among Gene clusters. Omics-a J. Integr. Biology. 16 (5), 284–287 (2012).
Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 31 (2), 455–461 (2010).
Moujaess, E., Kourie, H. R. & Ghosn, M. Cancer patients and research during COVID-19 pandemic: a systematic review of current evidence. Crit. Rev. Oncol. Hematol. 150, 102972 (2020).
Ribas, A. et al. Priority COVID-19 vaccination for patients with Cancer while Vaccine Supply is Limited. Cancer Discov. 11 (2), 233–236 (2021).
Dai, M. et al. Patients with Cancer Appear more vulnerable to SARS-CoV-2: a Multicenter Study during the COVID-19 outbreak. Cancer Discov. 10 (6), 783–791 (2020).
Tomazini, B. M. et al. Effect of dexamethasone on days alive and Ventilator-Free in patients with moderate or severe Acute Respiratory Distress Syndrome and COVID-19: the CoDEX Randomized Clinical Trial. JAMA. 324 (13), 1307–1316 (2020).
Zhang, M., Zhang, Y., Zhang, L. & Tian, Q. Mushroom polysaccharide lentinan for treating different types of cancers: a review of 12 years clinical studies in China. Prog Mol. Biol. Transl Sci. 163, 297–328 (2019).
Wang, J., Wang, H. Y., Xia, X. M., Li, P. P. & Wang, K. Y. Inhibitory effect of sulfated lentinan and lentinan against tobacco mosaic virus (TMV) in tobacco seedlings. Int. J. Biol. Macromol. 61, 264–269 (2013).
Philpott, D. J., Sorbara, M. T., Robertson, S. J., Croitoru, K. & Girardin, S. E. NOD proteins: regulators of inflammation in health and disease. Nat. Rev. Immunol. 14 (1), 9–23 (2014).
Sabbah, A. et al. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 10 (10), 1073–1080 (2009).
Liu, S. et al. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 347 (6227), aaa2630 (2015).
Udden, S. M. N. et al. NOD2 suppresses colorectal tumorigenesis via downregulation of the TLR pathways. Cell. Rep. 19 (13), 2756–2770 (2017).
Almeida-da-Silva, C. L. C., Savio, L. E. B., Coutinho-Silva, R. & Ojcius, D. M. The role of NOD-like receptors in innate immunity. Front. Immunol. 14, 1122586 (2023).
McGeachy, M. J., Cua, D. J. & Gaffen, S. L. The IL-17 family of cytokines in Health and Disease. Immunity. 50 (4), 892–906 (2019).
Coffelt, S. B. et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 522 (7556), 345–348 (2015).
Buja, L. M. et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc. Pathol. 48, 107233 (2020).
Cecchini, R. & Cecchini, A. L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses ;143. (2020).
Laforge, M. et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat. Rev. Immunol. 20 (9), 515–516 (2020).
Aikawa, N. & Kawasaki, Y. Clinical utility of the neutrophil elastase inhibitor sivelestat for the treatment of acute respiratory distress syndrome. Ther. Clin. Risk Manag. 10, 621–629 (2014).
Zeng, W., Song, Y., Wang, R., He, R. & Wang, T. Neutrophil elastase: from mechanisms to therapeutic potential. J. Pharm. Anal. 13 (4), 355–366 (2023).
Szturmowicz, M. & Demkow, U. Neutrophil Extracellular traps (NETs) in severe SARS-CoV-2 Lung Disease. Int. J. Mol. Sci. ;22(16). (2021).
Middleton, E. A. et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 136 (10), 1169–1179 (2020).
Cavalcante-Silva, L. H. A. et al. Neutrophils and COVID-19: the road so far. Int. Immunopharmacol. 90, 107233 (2021).
Masso-Silva, J. A. et al. Increased peripheral blood neutrophil activation phenotypes and Neutrophil Extracellular trap formation in critically ill coronavirus Disease 2019 (COVID-19) patients: a Case Series and Review of the literature. Clin. Infect. Diseases: Official Publication Infect. Dis. Soc. Am. 74 (3), 479–489 (2022).
Lungu, C. N. & Mehedinti, M. C. Molecular motifs in vascular morphogenesis: vascular endothelial growth factor A (VEGFA) as the leading promoter of Angiogenesis. Int. J. Mol. Sci. ;24(15). (2023).
Lu, L. et al. Human RNase3 immune modulation by catalytic-dependent and independent modes in a macrophage-cell line infection model. Cell. Mol. Life Sci. 78 (6), 2963–2985 (2021).
Iba, T., Levy, J. H., Levi, M. & Thachil, J. Coagulopathy in COVID-19. J. Thromb. Haemost. 18 (9), 2103–2109 (2020).
Author information
Authors and Affiliations
Contributions
S. Z. and H. S. wrote the main manuscript text, H.S. prepared the figures and S.Z. prepared the tables. Alll authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, S., Sun, H. Prognostic model for gastric cancer patients with COVID-19 and network pharmacology study on treatment by lentinan. Sci Rep 14, 24645 (2024). https://doi.org/10.1038/s41598-024-76783-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-76783-2