Abstract
The Coronavirus disease 2019 (COVID-19) pandemic has caused significant global threats, as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is primarily transmitted through airborne droplets and bioaerosols. Healthcare workers are particularly at high risk, yet there is limited research on the presence of SARS-CoV-2 in bioaerosols within healthcare facilities in Malaysia. This study aimed to determine the presence and viability of SARS-CoV-2 and its variants of concern in the air and ventilation systems of designated COVID-19 facilities from December 2021 to February 2022. Samples were collected from two hospitals and one quarantine centre (QC), including medical wards, intensive care units, emergency departments, and QC halls. Air samples were obtained using air samplers, while surface samples were taken from return air grilles. SARS-CoV-2 ribonucleic acid (RNA) and its variants were detected using reverse transcription droplet digital polymerase chain reaction (RT-ddPCR) and PCR-based genotyping, respectively. Results showed that Hospital A had a higher rate (24.6%) of positive samples than Hospital B (8.8%). Surface samples had a higher positivity rate (50.0%) compared to air samples (8.3%). The detected variants included delta (34.7%), a mixture of delta and omicron (8.7%), non-variant of concern (non-VOC) (8.7%), and omicron (4.3%). This study emphasizes the need for strict airborne infection control measures for healthcare workers.
Similar content being viewed by others
Introduction
The Coronavirus disease 19 (COVID-19), a highly contagious and lethal coronavirus illness caused by the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has resulted in a significant impact on the public health systems worldwide. The high transmissibility and infectivity rate of the disease led the World Health Organization (WHO) to declare a worldwide pandemic on March 11, 20201, signifying a global public health crisis2. The WHO identified droplets and contact with infected persons or contaminated surfaces as the primary modes of transmission in the early stages of the pandemic3. However, since June 2020, a growing body of evidence has unequivocally demonstrated aerosols as the primary mode of transmission for this virus]. Evidence suggests airborne transmission as the spread of an infectious agent generated by the dispersion of droplet nuclei (aerosols) that remain infectious while suspended in the air7. The airborne transmission of SARS-CoV-2 has been reported even in the absence of aerosol-generating procedures that generate aerosols8. Super-spreading infections further suggest the role of aerosol transmission in the pandemic9,10. Aerosols which are smaller than droplets, are defined as substances smaller than 5 μm in diameter. They can remain airborne longer, enabling transmission at greater distances and longer durations11.
However, pathogenic viruses in bioaerosols are difficult to detect due to their low concentrations in the air8,12. Viruses are also more prone to inactivation during aerosol collection as compared to bacteria or fungi, although their susceptibility varies greatly depending on the collection technique and species13. Many studies have shown that healthcare workers are at risk of infection through contact with COVID-19 patients and nosocomial outbreaks14,15,16. Studies on environmental contamination of SARS-CoV-2 in hospital settings have shown the presence of viral ribonucleic acid (RNA) in air and ventilation openings such as return air grilles17,18,19,20.
As for surface detection, under controlled conditions, the virus has been shown to remain detectable and viable for several days on various surfaces21. The kinetics of viability decay directly correlate with increasing temperature and are also influenced by factors such as humidity, UV exposure, material type, and surface roughness21,22,23. Viable virus has been isolated from the air around infected patients, and viral RNA has been found in aerosol particles more than 1.5 m away from the source8,24,25. Poor ventilation in confined indoor spaces is linked to a higher transmission of respiratory infections. The detection of viruses in mechanical ventilation systems underscores the potential for airborne transmission, emphasizing the necessity of properly implementing ventilation systems to help protect against these infections4.
To the best of our knowledge, there remains a significant dearth of information concerning the presence of SARS-CoV-2 in bioaerosols within Malaysia healthcare settings. No previous research has been published on the presence of SARS-CoV-2 RNA in both air and surfaces of return air grilles in the wards and air handling units (AHUs). Therefore, this study aims to fill this gap by determining the presence of SARS-CoV-2 and its variant of concern as well as their viability in the air and surfaces of return air grilles in the designated COVID-19 treatment facilities. SARS-CoV-2 can easily spread to individuals through airborne transmission via droplets and aerosols, leading to outbreaks that are challenging to control. Thus, detecting the virus in bioaerosols is crucial for stakeholders to enforce effective infection control interventions, prevent outbreaks, and ensure healthcare workers’ safety.
Results
Detection of SARS-CoV-2 in bioaerosols and surface samples
A total of 156 environmental samples were collected from three selected designated COVID-19 facilities, consisting of 132 air samples and 24 surface samples. Only 23 samples were positive with a detection rate of 14.7%. The concentrations of viral gene copies recovered from each sample type (air and surface) varied widely between samples, ranging from non-detectable (ND) to 0.309 and ND to 16.00 for N2 and ND to 0.225 and ND to 0.676 E-gene copies/µl respectively. There were significant differences in the percentage of positive samples found across different types of healthcare facilities (p < 0.05) (Table 1). The highest detection was in Hospital A (24.6%) followed by quarantine centre (QC) (9.5%) and Hospital B (8.8%). We observed a positive detection in 19.7% of samples in the medical wards, 17.2% of samples in the intensive care unit (ICU), and 9.5% of samples in the QC hall but none in the emergency department (ED).
Air samples and surface swab samples from return air grilles revealed airborne viral shedding in these facilities as evidenced by the positive samples. A significant difference (p < 0.05) was found between types of sampling whereby 50.0% of surface swab samples were detected positive for SARS-CoV-2 RNA as compared to 8.3% of air samples. According to the type of air sampler, the results showed no significant association between the different types of air sampler and the detection rate (p > 0.05). The NIOSH fractionation technique detected 66.6% positive samples from both 1.5 mL and 15 mL tubes that captured particles with a size of > 1 μm. Meanwhile, another 33.3% were detected from filter paper that captured particle size of < 1 μm. Overall, Coriolis had a slightly higher positive detection rate at 11.4% compared to NIOSH at 6.8%. In this study, SARS-CoV-2 RNA was also detected in air samples at both 1 m and 2.5 m distances from COVID-19 patients. However, there was no significant difference (p > 0.05) between the SARS-CoV-2 RNA detection rate at 1 m and 2.5 m from the confirmed COVID-19 patients. Air sampling conducted at a 1-meter distance revealed gene copy ranges for the nucleocapsid 2 (N2) and envelope (E) genes from 0.073 to 0.309 copies/µl and ND (Not Detected) to 0.217 copies/µl, respectively. Similarly, sampling at a 2.5-meter distance showed copy numbers ranging from 0.075 to 0.107 copies/µl for the N2 gene and 0.088 to 0.225 copies/µl for the E gene, as detected by the respective assays. (Supplementary Table 1).
Effects of sample types on the presence and absence of SARS-CoV-2 detection
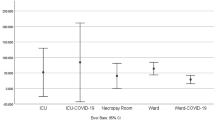
The detection of SARS-CoV-2, the virus responsible for COVID-19, is crucial for effective disease control and prevention. Two commonly used sample types for detecting the presence of SARS-CoV-2 are surface swabs and air samples. In this study, we determined the effect of these sample types on the positive detection (23 samples) of the virus using N2 and E gene assay. The SARS-CoV-2 RNA titers were detected in 100% (11/11) and 90.9% (10/11) of air samples, for N2 and E gene assays, respectively, while for the surface samples, the viral genome was detected in 100% (12/12) and 83.3% (10/12) for the two gene assays N2 and E respectively. Table 2 shows the SARS-CoV-2 RNA concentration descriptive statistics of air and surface samples by gene assay, complementing the information shown in Fig. 1.
Both sample types can generate representative and useful results for virus tracking and monitoring. However, the air sampling procedure must be well designed, considering the types of air samplers being used. The relationship between N2 and E genes for both sample types is shown in Fig. 2. The N2 and E data of the positive air and surface samples were plotted. Spearman’s rho correlation showed a significantly strong positive association between the N2 gene and the E gene, (r = 0.944, < 0.05), for surface samples. No significant correlation was observed for the air sample thus indicating that sample type produced different trends in gene data.
SARS-CoV-2 genotyping and viability analysis
A total of 23 positive samples were analysed for their genotypes. Genotyping detected approximately 34.7% (8/23) delta variants (N, P681R, L452R), 8.7% (2/23) mix of delta and omicron (N, N501Y, K417N, P681R, E484A, L452R), 8.7% (2/23) non-VOC (N-gene), and 4.3% (1/23) omicron variants (N501Y, K417N). A total of 7 samples were sent for tissue culture. However, all SARS-CoV-2 RNA tested was not viable through in-vitro inoculation.
Discussion
This study demonstrated the presence of SARS-CoV-2 in the air and surface swabs of a COVID-19 QC and two designated COVID-19 hospitals. The results were consistent with majority of other published studies. It was important to note that the proportion of positive viral RNA in both air and surface swab samples is comparatively lower than other studies17,18,19,20 possibly attributed by variations such as sampling strategy and methods, the environmental factors of the buildings and clinical spaces, patient characteristics, patient shedding, and disinfection protocol. Our study finds that, surface samples of SARS-CoV-2 are often more contaminated than air samples because of the cumulative effects and are influenced by environmental factors. SARS-CoV-2 can remain viable on various surfaces for hours to days, depending on temperature and humidity. Studies have shown that the virus can be isolated from surfaces even after several days, highlighting the risk of transmission through contact with contaminated surfaces, particularly in high-touch areas. Swab samples at AHUs can trap contaminants from multiple sources, leading to higher concentrations of viral material compared to the more dispersed nature of airborne particles. Additionally, these swab samples can capture larger amounts of the virus than air samples, which may dilute the viral particles26. Since SARS-CoV-2 RNA was discovered in the ventilation systems far from the treatment areas, it may suggest that the virus can travel a long distance, where droplet transmission alone is not sufficient to explain the situation. The findings of this study confirmed the WHO’s definition of aerosolized SARS-CoV-2 transmission, which states a positive detection rate of the SARS-CoV-2 RNA at a distance of > 2 m from the patient3.
Furthermore, the recovery of viral RNA from the surface of return air grilles and AHU requires detection not only > 2 m from a patient but also at a height that is inaccessible through direct contact implying that smaller-sized viruses may be displaced by airflows and deposited on equipment such as vents. Overall, these findings supported the concept of airborne transmission of SAR-CoV-2 which has been proven in earlier studies20,24,27,28. However, the findings contradict several other studies29,30,31,32 that reported all air samples as negative despite extensive environmental contamination. Those studies, however, used a small number of air samples and were conducted in rooms with high rates of dilution ventilation. A thorough understanding of airborne viral transmission as well as the ability to rapidly and accurately identify airborne viruses is important to protect healthcare workers. This is because infection prevention and control measures are linked to the spread of respiratory pathogens.
Detecting viruses in air samples poses significant challenges due to their low concentrations compared to other microorganisms. This necessitates sampling larger air volumes, measured in cubic meters, to ensure reliable analysis results, unlike the sampling of chemical agents. However, this study suggests that both low and high-volume samplers are helpful and may represent adequate strategies for detecting airborne SARS-CoV-2.
Air sampling using size-fractionation techniques is essential for a better understanding of the mode of viral transmission. The size of a virus-laden aerosol determines the sites where it can be deposited in the respiratory system. It is interesting to note that according to this study, the smallest size fraction with detectable levels of SARS-CoV-2 was < 1 μm with a positive detection rate of 70%. In contrast, all samples from the fractionated size < 1 μm in the study of air samples from airborne isolation rooms in a hospital in Singapore were negative18. The aerosol collected by the NIOSH BC-251 smallest stage (< 1 μm) corresponds to the smallest mode of exhaled breath particles that has the potential to travel to the lower respiratory tract33. Many studies on SARS-CoV-2 air sampling did not conduct particle size measurements17,24,32,34.
A study by Zhou et al.17 using a Coriolis µ air sampler detected 38.7% positive air samples in teaching hospitals in London. Another study by Chia et al., (2020)18 in Singapore using NIOSH-BC 251 detected a higher positivity rate of 66.7%. Additionally, all air samples collected using a NIOSH sampler were found to be positive in another study conducted by Ge et al. (2020)19 in China. Similarly, Guo et al. (2020)20 found that aerosols collected using Surface Air System Sampler 2300 (SASS 2300) showed detectable SARS-CoV-2 RNA in 28.6% of air samples as well as in surface swab samples at air outlets in both ICUs and general wards in Wuhan hospital. In contrast, some other research could not detect17,35,36,37 or did not test8,18,19,38 for the viability of the airborne virus. Furthermore, there are a limited number of studies that employed size-fractionation techniques to determine the size range of SARS-CoV-2 particles which suggest that viral particles are produced by individuals with COVID-19 infections39. While significant research has been conducted on SARS-CoV-2 transmission and genomic surveillance in clinical settings, fewer studies have explored genomic sequencing from environmental samples.
This study also highlighted that both the positive detection rate and viral load level were higher in the medical wards than in the ICU, QC, or ED. This suggests that the likelihood of transmission is higher in the medical wards, indicating better infection prevention control and practice in the ICU, QC, and ED despite patients undergoing aerosol-generating procedures in these locations. However, an important factor that may have contributed to this result is the consistently lower number of patients in the ICU and a lower patient to ward ratio compared to the medical wards. The medical wards has higher turnover of COVID patients and most patients are more mobile, potentially leading to wider spread of viral shedding in the area. On the contrary, a study at Huoshenshan Hospital in Wuhan, the epicenter of the outbreak, found higher contamination in the ICU compared to the general ward20. The results of another study also showed that viral shedding was higher in ICU due to higher viral load in patients requiring prolonged ICU stay compared to patients in the general ward40. Furthermore, the study reported that the broncho-alveolar lavage fluid (BALF) aspirated from patients requiring invasive mechanical ventilation had the highest SARS-CoV-2 load40. Nevertheless, this result should be interpreted with caution due to the smaller number of samples taken from the ICU compared to the ward. Meanwhile, the quarantine centre hall, being a much larger space, had a reduced potential for localized viral accumulation. While for ED, the patients triaged were mixed with other non-COVID patients, potentially reducing the viral load.
It is significant to note that this study highlighted the importance of analysing more than one target to avoid false-negative results. Our study showed that all (100%) of the samples in which SARS-CoV-2 was detected in air and surface samples tested positive for the N2 gene, but only 90.9% and 83.3% of the signal was detected for the E target gene. Due to the potential for the rapid spread of infection in healthcare settings, it is important that even at low concentrations, the virus is detected to act as an early warning monitoring system, allowing the healthcare facilities to implement public health measures to reduce the transmission and spread. Therefore, it is recommended that a minimum of two SARS-CoV-2 genetic targets be measured to reduce the probability of false negatives.
To our knowledge, limited research has previously been conducted globally to evaluate genome sequences from environmental samples on SARS-CoV-2. Based on the PCR-based genotyping, we identified Delta, a mix of Delta and Omicron, non-VOC (N-gene), and Omicron variants in our air and surface samples from both return air grilles in the wards, hall, and air handling unit. The SARS-CoV-2 strain that was isolated from air samplers and surface samples during the sampling period was the same as the wave that was reported41.
Where the viability of SARS-CoV-2 is concerned, a limited number of studies to date have recovered viable SARS-CoV-2 from environmental samples. Studies conducted by Lednicky et al. (2020)24 and Santarpia et al. (2020)37 using a virus identification via air sampling (VIVAS) air sampler showed viable SARS-CoV-2 in aerosols that could transmit a distance of > 2 m in the absence of aerosol-generating procedures in a hospital room. Yet, despite the optimisation of sampling methods, the use of Reverse Transcription Droplet Digital polymerase chain reaction (RT-ddPCR) that can detect targets in specimens with very low concentrations, and a significant proportion of samples being positive, there is no viable SARS-CoV-2 in our cell culture was isolated from surface swab and air samples in this study. Consequently, the possibility of our samples infecting a healthy individual is therefore unknown. Our findings are in line with the findings of other studies. For instance, in Canada Kotwa et al. 202136 examined the viability of positive samples with a Ct value on PCR analysis of < 34 and reported that none of the positive air samples yielded viable viruses using NIOSH cyclone sampler. Other studies also discovered non-viable viruses, including those conducted in Singapore using Biospot-VIVAS BSS300P air sampler35 and in London using Coriolis17.
The difficulty in isolating viruses from air samples can be attributed to several factors such as a combination of its low viral concentrations, the effects of air sampling methods on the viral cell membrane, surface protein integrity18, viral dehydration during the collection process, viral damage due to impact forces, re-aerosolization of viral during collection, loses due to the viral retention at the samplers’ inputs or walls34 and in vitro cell culture infection method32.
Characteristic mutations, key mutations, or signature mutations play an important role in the analysis of air and surface swab samples. They serve as the basis for the development of PCR-based assays42. In this study, all characteristic mutations occurred in the S gene. During the sampling period from December 2021 to February 2022, there was a noticeable trend of characteristic mutations in both air and surface swab samples. In December 2021 and January 2022, the Delta variant and a mixture of Delta and Omicron were observed which concurred with another study from Malaysia that revealed co-circulation of Delta and Omicron from Jan-Feb 202243. However, in February, genotyping revealed a shift in characteristic mutations, with a predominant presence of the Omicron variant. This finding aligns with a study by Azami et al. (2022)44, which also indicated similar changing trends in circulating SARS-CoV-2 lineages in Malaysia. Over the surveillance period from June to December 2021, the Delta variant was the dominant circulating variant, and eventually the Omicron variant displaced the Delta variant by January 202244.
In summary, this study provides evidence that SARS-CoV-2 was present in the air and return air grilles of the rooms where COVID-19 patients are housed and cared for. Whilst this study did not confirm the viability, it did suggest the potential for bioaerosol transmission. However, it is noteworthy that the presence of SARS-CoV-2 RNA in aerosols does not correlate with virus viability or inhalation risk, as non-infectious SARS-CoV-2 cannot cause COVID-19. Prioritizing the implementation of infection prevention and control measures at work may be the key to controlling the COVID-19 pandemic and containing this highly infectious disease to ensure the health and safety of healthcare workers. One of the most important measures is to maximize ventilation through engineering controls combined with regular disinfection and the use of appropriate personal protective equipment.
Strengths and limitations
Our study has noteworthy strengths which include variation in sampling methodology, concurrent air and surface sampling, and detection and quantification of virus was done by RT-ddPCR which is a sensitive assay that could detect low viral load as in environmental samples. In addition, we perform PCR-based genotyping on all SARS-CoV-2 isolates to identify the type of variants, and finally we proceed with culture to ascertain the virus viability.
There are a few limitations to our study. Firstly, the aerosol sampling was only performed on one patient for a duration of 10 min and 4 h using Coriolis and NIOSH, respectively. This was done at a single time point during the course of the illness. Secondly, although the patient had an upper respiratory infection and tested positive for COVID-19, we were unable to determine whether or not the patient was actively shedding the virus on the day of the sampling as we did not collect samples from the patient. Additionally, it is possible that the sample was too diluted and the RNA concentration was below the detection threshold. Since clinical results considered were within 72 h of environmental testing, it is possible that the viral load was low or negligible on the testing day, hence limiting environmental contamination. Environmental factors such as ventilation, air movement, relative humidity, and temperature within the study locations might affect the sampling and viral detection18. Thirdly, we were unable to verify that viable viruses are present which would be crucial in determining the infectiousness of the disease. Finally, this study was conducted over a short period of three months, during the final phase of the COVID-19 pandemic in Malaysia, just prior to the transition to the endemic phase. During these phases, changes in clinical management led to a significant reduction in hospital admissions, along with the closure of quarantine centers. Furthermore, the diagnostic methods shifted from PCR testing to a combination of PCR and/ or nasopharyngeal swab antigen tests. Thus, Ct value data are unavailable for all patients, making it impossible to correlate the Ct values with the percentage of positive results.
More study is anticipated to establish factors that control SARS-CoV-2’s emission as well as the factors that affect the successful detection of viable SARS-CoV-2 from air samples and the use of enhanced virus culture techniques.
Methods
Bioaerosol sampling
Two types of air samplers, the National Institute for Occupational Safety and Health (NIOSH) BC-251 (Morgantown, WV, USA) two-stage cyclone sampler, and Coriolis µ (Grade EPM 2000, 47 mm, Whatman®, USA) were used. For each sampling day, two NIOSH BC-251 a 2-stage cyclone sampler, and one Coriolis were used in the COVID-19 patient room. Each air sampler was mounted on a tripod above the floor level, about the height of the patient’s head positioned approximately 1.0 and 2.5 m away from the COVID-19 patients. The COVID-19 patients in the selected room were not required to wear face masks. The NIOSH air sampler was connected to the SKC AirCheck TOUCH pump, set to a flow rate of 3.5 L/min, and operated for four hours during each sampling period. The NIOSH sampler collected particles in three size fractions: particles with a diameter > 4 μm were collected in a 15 mL centrifuge tube, 1–4 μm were collected in a 1.5 mL centrifuge tube, and < 1 μm were collected in a 37 mm diameter 2 μm pore-size polytetrafluoroethylene (PTFE) filter cartridge. The Coriolis µ air sampler collected air samples in conical tubes containing 5mL of viral transport medium (VTM) and processed them for 20 min at a flow rate of 100 L/min. This air sampler used liquid cyclone technology to collect particles as small as 0.5 μm in diameter. The device inlet was positioned facing the COVID-19 patient’s face.
All sample tubes were put in a self-sealing bag and the outer part of the sealed bag was cleaned with 80% isopropyl alcohol solution before leaving the sampling area and sealed with another self-sealing bag. Samples were then transferred to the laboratory on the same day at 4oC. To avoid direct heating, the membrane filter collected in the cartridge was wrapped in aluminium foil. It was then placed in a self-sealing bag cleaned with 70% ethanol solution before placing it in the transport container. A set of control samples was collected on each sampling day by placing the sample tube into the machine without drawing the air in. Control samples were treated the same as air samples from the patient care areas. Both air samplers were decontaminated after each experiment as per the manufacturer’s instructions. The sample preparation procedures were conducted following the methodology described by Rashid et al. (2023)45.
Surface sampling
Surface samples were obtained by swabbing the return air grilles in the designated wards and AHU using a sterile nylon-flocked swab pre-moistened with sterile VTM. The swab was immersed in VTM and wiped in an overlapping “S” pattern, first horizontally followed by vertically and then diagonally, while rotating with gentle pressure over a recommended surface area of 25 cm2, following the WHO sampling guidelines46. The moist swabs were promptly transferred to a vial containing 1 ml of VTM. The collected VTMs were wrapped in absorbent material and placed in a self-sealing bag. Before being placed in the transport container, the self-sealing bag was cleaned with a 70% ethanol solution. All samples were stored at temperatures between 2 and 8 °C and transported to the laboratory within 4 h. The sample preparation procedures were conducted following the methodology described in Rashid et al. (2023)45. Control samples were treated similarly to the swab samples from the potentially contaminated areas, including opening the package and removing the swab from the tube, but without sampling any surfaces. All personnel involved in both environmental sampling wore full PPE and complied with the Ministry of Health (MOH) Malaysia guidelines for healthcare workers to prevent infection and sample contamination.
RNA extraction and concentration
Before RNA extraction, the samples were heated in a thermomixer for 30 min at 65 °C. The QIAamp® Viral RNA Mini Kit (Qiagen, Hilden, Germany) was then used as specified by the manufacturer with minimal modifications. A large input sample volume (420 µl) was used. The amount of lysis buffer and other reagents added to the samples was increased proportionately, while the amount of buffers AW1 and AW2 used in the washing stages remained constant. A double elution using 2 × 40 µl buffer AVE was performed to elute the isolated RNA. The extracted RNA was duplicated and kept a −80 °C for further amplification45.
RT-ddPCR
We used the Centers for Disease Control and Prevention (CDC) nucleocapsid 2 (N2) and envelope (E) primer-probe sets from the 2019-nCoV CDC ddPCR Triples Probes and the One-Step RT-ddPCR Advanced Kit for Probes from Bio-Rad, United States to achieve absolute quantification of SARS-CoV-RNA in the samples. Standard PCR mixtures with 20% overage for each sample were 10.8 µl of RNA template, 2.4 µl of one-step reverse transcriptase, 1.2 µl of 300 mM Dithiothreitol (DTT), 1.2 µl of RT-ddPCR triplex assay, and 8.4 µl of nuclease-free water, as recommended by the manufacturer. The total volume of each reaction mixture was 24 mL. To reduce reagent contamination, mixtures were prepared in a pre-PCR room. A 20 mL reaction mixture was used to generate droplets with a Bio-Rad droplet generator according to the manufacturer’s instructions. The resulting emulsion was then transferred to a new 96-well plate (catalog number 951020389, Eppendorf, Enfield) for PCR amplification. The PCR protocol was as follows: Reverse transcriptase (RT) was performed at 50 °C for 60 min, followed by polymerase activation at 95 °C for 10 min, 40 cycles of denaturation at 94 °C for 30 s, and annealing/extension at 55 °C for 60 s. Droplets were stabilized at 4 °C for 30 min after inactivating the polymerase at 98 °C for 10 min. Droplet quantification was done using a QX Manager Software Standard Edition, v 1.2. Samples were deemed non-quantifiable for analysis if the average number of partitions was < 10,000 accepted droplets. For N2 and E primers and probe sets, the positive threshold for detecting SARS-CoV-2 RNA through RT-ddPCR was determined to be 0.07 copies/µl or higher. An outcome of below 0.07 copies/µl for both N2 and E primers/probe sets was interpreted as negative. The method for RT-ddPCR was adapted from the study by Rashid et al. (2023)45.
Variant genotyping
Samples positive by the RT-ddPCR assay were genotyped for the SARS-CoV-2 variant using a commercial RT-PCR assay. The RNA extracts were aliquoted prior to the molecular testing workflow of this study to prevent repetitive freezing and thawing. Genotyping was carried out using the Powercheck SARS-CoV-2 S-gene Mutation Detection Kit Ver. 3 (Kogene Biotech, Korea) on a CFX-96 Thermal Cycler (Biorad, USA) in accordance with the manufacturer’s instructions. The kit consists of two sets of primer/probe mixes (P1 and P2), two sets of controls (C1 and C2), and an RT-PCR master mix. Two sets of probes can detect SARS-Cov-2 together with seven variants of the S gene: N501Y, K417N, P681R, E484A/K, T547K and L452R. By combining these variants, five SARS-CoV-2 variants consisting eight sub-lineages could be screened. Briefly, the Alpha (B.1.1.7) variant is implied when just N501Y is detected. Detecting N501Y, K417N, and E484K indicate the Beta (B.1.351) variant. Detecting N501Y and E484K implies the Gamma (P.1) variant. Detecting P681R and L452R suggest the Delta (B.1.617.2, AY.1 & AY.2) variant while T547K and E484A combination identifies the Omicron (BA.1 & BA.2) variant. The assay requires FAM, JOE (VIC or HEX), ROX, Cy5 and Quasar705 amplification channels. The controls provided in the assay included an exogenous RNA control (ERC) and an internal control (GAPDH).
Viability test
Viral isolation was performed on positive environmental samples by RT-ddPCR to assess the viability. Since SARS-CoV-2 was a class 3 pathogen, viral isolation was conducted in a biosafety level 3 (BSL-3). Vero E6 cells were grown overnight in Dulbecco’s Modified Eagle’s Medium (DMEM) (Gibco, US) supplemented with 10% fetal bovine serum (FBS) (Gibco, USA) in a 6-well plate (Thermo Fisher Scientific, USA). During virus inoculation, the media in each well was replaced with 200 µL of virus sample in VTM, and incubated at 37 °C with 5% CO2 on a tilted Ultrarocker Rocking Platform for 30 min (Bio-Rad, USA). The inoculated cells were then washed with 1x Phosphate Buffered Saline (PBS) to remove unbound viruses. The infected cells were finally maintained in 2mL of DMEM supplemented with 2% FBS in a 37 °C incubator with 5% CO2. The cytopathic effect (CPE) was monitored for seven days which is typically represented by shrinking, sloughing, lysis and detachment of the infected cells. If clear CPE was observed, culture supernatants were collected and analyzed for SARS-CoV-2 growth by determining and comparing the pre and post inoculation cycle threshold (Ct) by RT-PCR.
Statistical analysis
Data analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 28 (SPSS Inc. Chicago). Crosstabulation was applied to assess the positivity rate of samples based on facilities, locations, sample types, air sampler types, and distance. A Chi-squared test was performed to assess the association between each categorical variable and the positive detection rate with results with a p-value < 0.05 considered significant. Spearman’s correlation was performed to test the associations between the N2 and E gene detection in the air and surface samples. Data visualisations using dot plots for the N2 and E genes from the samples were depicted.
Ethics statement
The study protocol was evaluated by the Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia (MOH). As the study did not fall within the scope of the Malaysian Act on Medical Research Involving Human Subjects no further medical ethical approval was required. Ethical approval of this study was received from the Medical Research and Ethics Committee [KKM/NIH/P21-1159 (4)]. Throughout the research, all methods were carried out with the highest ethical considerations and in accordance with relevant guidelines and regulations—Guidelines for Conducting Research in Ministry of Health Institutions and Facilities.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
1. WHO Director-General’s opening remarks at the media briefing on COVID-19. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (2020).
2. Sharma, A., Tiwari, S., Deb, M. K. & Marty, J. L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): a global pandemic and treatment strategies. Int J Antimicrob Agents. 56, https://doi.org/10.1016/j.ijantimicag.2020.106054 (2020).
3. Mask use in the context of COVID-19. https://www.who.int/publications/i/item/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak (2020).
4. Nissen, K. et al. Long-distance airborne dispersal of SARS-CoV-2 in COVID-19 wards. Sci Rep. 10, https://doi.org/10.1038/s41598-020-76442-2 (2020).
5. Dubey, A. et al. Evidence of the presence of SARS-CoV-2 virus in atmospheric air and surfaces of a dedicated COVID hospital. J Med Virol. 93, 5339–49; https://doi.org/10.1002/jmv.27029 (2021).
6. Tang, S. et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ Int. 144, 106039; https://doi.org/10.1016/j.envint.2020.106039 (2020).
7. World Health Organization. Pandemic and Epidemic Diseases, World Health Organization. Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care: WHO guidelines. https://apps.who.int/iris/bitstream/handle/10665/112656/9789241507134_eng.pdf;jsessionid=41AA684FB64571CE8D8A453C4F2B2096?sequence=1 (2014).
8. Liu, Y. et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 582, 557–60; https://doi.org/10.1038/s41586-020-2271-3 (2020).
9. Lu, J. et al. COVID-19 Outbreak associated with air conditioning in restaurant, Guangzhou, China. Emerg Infect Dis. 26,1628–31; https://doi.org/10.3201/eid2607.200764 (2020).
10. Rocklöv, J., Sjödin, H. & Wilder-Smith, A. COVID-19 outbreak on the diamond princess cruise ship: Estimating the epidemic potential and effectiveness of public health countermeasures. J Travel Med. 27, 1–7; https://doi.org/10.1093/JTM/TAAA030 (2021).
11. van Doremalen N. et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 382 (16), 1564–1567; https://doi.org/10.1056/NEJMc2004973 (2020).
12. Borges, J. T., Yuri, L., Nakada, K., Guedes Maniero, M. & Guimarães, J. R. SARS-CoV-2: a systematic review of indoor air sampling for virus detection. Environ Sci Pollut Res Int. 28 (30), 40460–40473; https://doi.org/10.1007/s11356-021-13001-w (2021).
13. Bhardwaj, J. et al. Recent advancements in the measurement of pathogenic airborne viruses. J Hazard Mater. 420, https://doi.org/10.1016/j.jhazmat.2021.126574 (2021).
14. Cheng, V. C. C. et al. Nosocomial outbreak of Coronavirus disease 2019 by possible airborne transmission leading to a superspreading event. Clinical Infectious Diseases. 73, E1356–64; https://doi.org/10.1093/cid/ciab313 (2021).
15. Lim, W. Y. et al. First nosocomial cluster of COVID-19 due to the Delta variant in a major acute care hospital in Singapore: investigations and outbreak response. J Hosp Infect. 122, 27–34; https://doi.org/10.1016/j.jhin.2021.12.011 (2022).
16. Sakamoto, N. et al. Nosocomial outbreak of coronavirus disease in two general wards during the initial wave of the pandemic in 2020, Tokyo, Japan. Western Pac Surveill Response J. 13, 1–5; https://doi.org/10.5365/wpsar.2022.13.1.906 (2022).
17. Zhou, J. et al. Investigating severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) surface and air contamination in an acute healthcare setting during the peak of the Coronavirus disease 2019 (COVID-19) pandemic in London. Clin Infect Dis. 73, E1870–7; https://doi.org/10.1093/cid/ciaa905 (2021).
18. Chia, P. Y. et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun. 11, https://doi.org/10.1038/s41467-020-16670-2 (2020).
19. Ge, X. Y. et al. Evaluation of the exposure risk of SARS-CoV-2 in different hospital environment. Sustain Cities Soc. 61, https://doi.org/10.1016/j.scs.2020.102413 (2020).
20. Guo, Z. D. et al. Aerosol and surface distribution of severe acute respiratory syndrome Coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 26, 1586–91; https://doi.org/10.3201/eid2607.200885 (2020).
21. Noorimotlagh, Z., Jaafarzadeh, N., Martinez, S. S. & Mirzaee, S. A. A systematic review of possible airborne transmission of the COVID-19 virus (SARS-CoV-2) in the indoor air environment. Environ. Res. 193, 110612; https://doi.org/10.1016/j.envres.2020.110612 (2021).
22. Kampf, G., Todt, D., Pfaender, S. & Steinmann, E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect. 104, 246–251; https://doi.org/10.1016/j.jhin.2020.01.022 (2020).
23. Jarvis, M. C. Aerosol transmission of SARS-CoV-2: Physical principles and implications. Public Health Front. 8, 590041; https://doi.org/10.3389/fpubh.2020.590041 (2020).
24. Lednicky, J. A. et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int J Infect Dis. 100, 476–82; https://doi.org/10.1016/j.ijid.2020.09.025 (2020).
25. Setti, L. et al. Airborne transmission route of COVID-19: Why 2 meters/6 feet of inter-personal distance could not be enough. Int J Environ Res Public Health. 17, 2932; https://doi.org/10.3390/ijerph17082932 (2020).
26. Correia, G. et al. SARS-CoV-2 air and surface contamination in residential settings. Sci. Rep. 12, 18058; https://doi.org/10.1038/s41598-022-22679-y (2022).
27. Rufino de Sousa, N. et al. Detection and isolation of airborne SARS-CoV-2 in a hospital setting. Indoor Air. 32, https://doi.org/10.1111/ina.13023 (2022).
28. Krambrich, J. et al. SARS-CoV-2 in hospital indoor environments is predominantly non-infectious. Virol J. 18, https://doi.org/10.1186/s12985-021-01556-6 (2021).
29. Liu, W. et al. Environmental contamination with SARS-CoV-2 in COVID-19 hospitals in Wuhan, China, 2020. Environ Microbiol. 23, 7373–81; https://doi.org/10.1111/1462-2920.15695 (2021).
30. Ong, S. W. X. et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 323, 1610–2; https://doi.org/10.1001/jama.2020.3227 (2020).
31. Kim, U. J. et al. Air and environmental contamination caused by COVID-19 patients: A multi-center study. J Korean Med Sci. 35, https://doi.org/10.3346/JKMS.2020.35.E332 (2020).
32. Chan, V. W. M. et al. Air and environmental sampling for SARS-CoV-2 around hospitalized patients with coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol. 41, 1258–65; https://doi.org/10.1017/ice.2020.282 (2020).
33. Santarpia, J. L. et al. The size and culturability of patient-generated SARS-CoV-2 aerosol. J Expo Sci Environ Epidemiol. 32, 706–11; https://doi.org/10.1038/s41370-021-00376-8 (2022).
34. Pan, M., Lednicky, J. A. & Wu, C. Y. Collection, particle sizing and detection of airborne viruses. J Appl Microbiol. 127, 1596–611; https://doi.org/10.1111/jam.14278 (2019).
35. Ong, S. W. X. et al. Lack of viable severe acute respiratory coronavirus virus 2 (SARS-CoV-2) among PCR-positive air samples from hospital rooms and community isolation facilities. Infect Control Hosp Epidemiol. 42, 1327–32; https://doi.org/10.1017/ice.2021.8 (2021).
36. Kotwa, J. D. et al. Surface and air Contamination with severe acute respiratory syndrome Coronavirus 2 from hospitalized Coronavirus disease 2019 Patients in Toronto, Canada, March-May 2020. J Infect Dis. 225, 768–76; https://doi.org/10.1093/infdis/jiab578. (2022).
37. Santarpia, J. L. et al. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci Rep. 10, https://doi.org/10.1038/s41598-020-69286-3 (2020).
38. Lei, H. et al. SARS-CoV-2 environmental contamination associated with persistently infected COVID-19 patients. Influenza Other Respir Viruses. 14, 688–99; https://doi.org/10.1111/irv.12783 (2020).
39. Stern, R. A., Al-Hemoud, A., Alahmad, B. & Koutrakis, P. Levels and particle size distribution of airborne SARS-CoV-2 at a healthcare facility in Kuwait. Sci Total Environ. 782, https://doi.org/10.1016/j.scitotenv.2021.146799 (2021).
40. Chen, P. F. et al. Virus load and virus shedding of SARS-CoV-2 and their impact on patient outcomes. World J Clin Cases. 8 (24), 6252–6263; https://dx.doi.org/10.12998/wjcc.v8.i24.6252 (2020).
41. GELOMBANG VARIAN OMICRON _ COVID-19 MALAYSIA. https://covid-19.moh.gov.my/semasa-kkm/2022/02/gelombang-varian-omicron (2022).
42. X Reponen, T. Sampling for microbial determinations. in Exposure to microbiological agents in indoor and occupational environments (eds. Viegas, C., Viegas, S., Gomes, A., Täubel, M. & Sabino, R.) 85–96 (Springer, 2017).
43. Tan, K. K. et al. Continuous introduction, dispersal and replacement of SARS-CoV-2 variants in Kedah, Malaysia. Int J Infect Dis. 134, S1-S19; https://doi.org/10.1016/j.ijid.2023.05.020 (2023).
44. Azami, N. A. M. et al. SARS-CoV-2 genomic surveillance in Malaysia: displacement of B.1.617.2 with AY lineages as the dominant Delta variants and the introduction of Omicron during the fourth epidemic wave. Int J Infect Dis. 125, 216–226; https://doi.org/10.1016/j.ijid.2022.10.044 (2022).
45. Rashid, S. A. et al. (2023) Droplet digital PCR application for the detection of SARS-CoV-2 in air sample. Front Public Health. 11, 1208348; https://doi.org/10.3389/fpubh.2023.1208348 (2023).
46. Surface sampling of coronavirus disease (COVID-19): A practical “how to” protocol for health care and public health professionals. https://www.who.int/publications/i/item/surface-sampling-of-coronavirus-disease-(-covid-19)-a-practical-how-to-protocol-for-health-care-and-public-health-professionals (2020).
Acknowledgements
The authors would like to thank the Director General of Health Malaysia for the permission to publish this paper, and the Director of Institute for Medical Research for her support toward the development of article. We especially would like to thank all the healthcare workers from all hospitals and QC who helped in our study. We also acknowledge the National Institutes of Health (NIH), Ministry of Health Malaysia for approving the research protocol under the code NMRR-21-1002-60174.
Author information
Authors and Affiliations
Contributions
R.I., K.R., N.M.: conceptualisation, methodology; R.M.R.: project administration, methodology, acquisition, formal analysis, investigation, writing-original draft, visualisation; R.S., J.S.: supervision, resources; N.A.K.H, N.M.N.H, M.A.P., F.A.S: preparation of materials and data collection; S.A.R., R.N., AS. S.R.L.M., N.A.M.H: performed the laboratory works and lab analysis. The final manuscript was read and revised by all authors, who also gave their approval.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
The Medical Research and Ethics Committee (MREC), Ministry of Health Malaysia, granted approval for this study with reference number KKM/NIH/P21-1159 (4).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Robat, R.M., Nazakat, R., Rashid, S.A. et al. Detection of SARS-CoV-2 in bioaerosols and surface samples from healthcare facilities in Klang Valley, Malaysia. Sci Rep 15, 7192 (2025). https://doi.org/10.1038/s41598-025-91566-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-91566-z