Shutterstock.com
Community pharmacies could be commissioned to dispense COVID-19 oral antivirals by integrated care boards (ICBs), following draft guidance from NHS England.
The guidance, dated 30 September 2022 and seen by The Pharmaceutical Journal, aims to “assist ICBs in establishing and maintaining timely access to COVID-19 therapeutics during the remainder of 2022/2023 in preparation for routine provision from April 2023”.
In December 2021, NHS England opened COVID Medicine Delivery Units (CMDUs) across the country to administer COVID-19 treatments, including neutralising monoclonal antibodies (nMABs) and oral antivirals, to non-hospitalised vulnerable patients.
However, the guidance for ICBs states that as “the NHS moves from a pandemic to an endemic response to COVID-19 infections, ICBs will be at the forefront of providing timely access to COVID-19 therapeutics to their local populations”, adding that the “preferred route of access” to the treatments will be “through primary care and integrated urgent care”.
“The service will need to ensure timely access to different treatments options as they become available. The service will therefore need to provide an intravenous infusion service for treatment of patients with nMABs and antivirals [and] enable oral antiviral medicines to be dispensed by local community pharmacy and/or hospital pharmacy,” the guidance says.
As part of the ICB-led services, community pharmacies would be required to “dispense and deliver oral antiviral medicines as soon as possible after receipt of prescription to meet the window of treatment efficacy”.
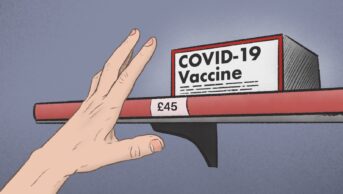
Community pharmacies in Scotland were commissioned to supply oral antiviral treatments to high-risk COVID-19 patients in January 2022, with a payment of £45 per item dispensed and an additional £12 to cover the cost of home delivery when needed.
In November 2021, molnupiravir became the first oral antiviral to be approved by the Medicines and Healthcare products Regulatory Agency (MHRA) for the treatment of COVID-19. This was followed by the approval of Paxlovid (nirmatrelvir/ritonavir; Pfizer) in January 2022, which was later recommended as a first-line treatment for eligible COVID-19 patients.
The draft guidance also noted that the National Institute for Health and Care Excellence has “commenced a Multi Technology Appraisal to review the clinical and cost effectiveness of currently available COVID-19 treatments in the longer term, which is expected read out early in 2023”.
According to the latest interim clinical commissioning policy, published on 30 May 2022, molnupiravir has conditional marketing authorisation for use in the treatment of mild to moderate COVID-19 in patients aged 18 years and over with a positive COVID-19 test, and who have at least one risk factor for developing severe illness.
Paxlovid has conditional marketing authorisation for the treatment of COVID-19 in adults who do not require supplemental oxygen and who are at increased risk for progression to severe COVID-19.
A spokesperson for NHS England confirmed that it “is in early discussions with ICBs on planning for the transition away from pandemic-specific arrangements to delivering routine access for COVID-19 treatments in the future”.
“As part of this planning, we will also be speaking to pharmacy representatives on the role that community pharmacy might have in future arrangements,” they said.
The spokesperson added that any decisions on wider access will be informed by the latest evidence, including from the national PANORAMIC study, as well as the forthcoming NICE appraisal.