Abstract
Hypertension is one of the most common risk factors for COVID-19 clinical progression. The identification of plasma biomarkers for anticipating worse clinical outcomes and to better understand the shared mechanisms between hypertension and COVID-19 are needed. A hypothesis-generating study was designed to compare plasma proteomics and metabolomics between 22 hypertensives (HT) and 41 non-hypertensives (nHT) patients with the most unfavorable COVID-19 progression. A total of 43 molecules were significantly differed between HT (n = 22) and nHT (n = 41). Random Forest (RF) analysis identified myo-inositol, gelsolin and phosphatidylcholine (PC) 32:1 as the top molecules for distinguishing between HT and nHT. Plasma myo-inositol and gelsolin were higher (P = 0.03 and P = 0.02, respectively) and plasma PC 32:1 was lower (P = 0.03) in HT compared to nHT. Biological processes like stress response and blood coagulation, along with KEGG pathways including ascorbate and aldarate metabolism (P = 0.021) and linoleic acid metabolism (P = 0.028), were altered in hypertensive patients with the most unfavorable COVID-19 progression. There is a clear link between hypertension and severe COVID-19. Key biological pathways to consider for improving the prognosis and quality of life of hypertensive patients who become infected with SARS-CoV-2 include oxidative stress, ascorbate and aldarate metabolism, lipid metabolism, immune system and inflammation.
Similar content being viewed by others
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly transmissible and pathogenic virus responsible for coronavirus disease (COVID-19)1. Hypertension is the most common preexisting condition in symptomatic COVID-19 patients that significantly increases the risk of hospitalization and death2,3,4,5,6,7,8. And although the exact mechanisms that link high blood pressure (BP) and COVID-19 severity remain unclear, the renin-angiotensin-aldosterone system (RAAS) dysregulation may be a key factor9. During SARS-CoV-2 infection, the viral spike protein binds to the angiotensin-converting enzyme 2 (ACE2) receptor, triggering the activation of a non-conventional RAAS axis. RAAS acts as a homeostatic regulator of vascular function. Activation of the conventional and non-conventional RAAS axis downregulates the ACE2 receptor, contributing to endothelial dysfunction and inflammation10,11. Consequently, the coexistence of SARS-CoV-2 infection and hypertension may have additive harmful effects on the patient12.
Some studies have reported specific biomarkers for the identification of COVID-19 patients at higher risk of adverse outcomes13, especially for those with cardiovascular complications14,15,16. Previous multi-omics analyses have advanced in the field of COVID-19 to support the prevention, diagnosis and treatment of disease severity17,18,19,20,21. However, there is a lack of biomarker-finding studies specifically focused on hypertensive patients with SARS-CoV-2 infection. For that reason, we evaluated the proteomic and metabolomic profiles of hypertensive patients in the acute phase of COVID-19 (hospital admission) stratified by disease severity. Our goal is to enhance our understanding of the mechanisms behind health decline, identify biomarkers for predicting worse clinical outcomes, and discover potential therapeutic targets.
Methods
Study cohort
The multi-omics study was performed in a cohort including 103 patients with SARS-CoV-2 infection confirmed by polymerase chain reaction (PCR) within the first 21 days of infection at the Hospital Universitari Joan XXIII of Tarragona (Spain). Patients were recruited between March 2020 and February 2021 (from the first to the third pandemic waves). COVID-19 patients were classified into three groups of severity according to the inclusion criteria described in “Diagnosis and Treatment Protocol for COVID-19 Patients (version 8 trial)”22,23: mild for patients that not require hospitalization, without symptoms or presenting mild symptoms such as fever, dry cough, headache, asthenia, anosmia, diarrhea, fatigue and myalgia; severe for hospitalized patients presenting with fever, mild/moderate pneumonia, mild/moderate dyspnea, or other pathologies developing severe symptoms; and critical for hospitalized patients that required intensive care and mechanical ventilation due to severe pneumonia, respiratory failure, hemodynamic instability, tachypnea ≥ 30/min, O2 saturation ≤ 93%, PaO2/FiO2 ≤ 300, lung infiltrates ≥ 50% radiological fields in 24–48 h, septic shock, or multi-organ dysfunction/failure. Among these 103 COVID-19 patients, 26 had hypertension and 77 were normotensives, as defined by the World Health Organization (systolic blood pressure readings ≥ 140 mmHg and/or diastolic pressure readings ≥ 90 mmHg on two different days)24. Hypertension diagnosis was documented in each patient’s medical history. All patients with hypertension except one (25/26) were receiving antihypertensive medication.
None of the patients in the study had received the SARS-CoV-2 vaccine at the time of blood sampling. Data was collected and stored in a database, including data related to hospitalization, such as symptoms at admission, radiological findings, pneumonia severity, oxygen therapy, medical treatment, comorbidities, biochemical and demographic data, and previous diseases of interest.
Sample recruitment
Blood samples were extracted on admission and processed to obtain plasma for multi-omics analysis. Plasma samples were stored at -80ºC at the BioBank of Institut d’Investigació Sanitària Pere Virgili (IISPV) until its use in the multi-omics analysis.
Proteomics
Sample preparation (12 μl) followed a protocol reported previously. Data acquisition was performed using an LTQ-Orbitrap Velos Pro from ThermoFisher by an enhanced FT-resolution MS spectrum 4 (R = 30,000 FHMW) followed by a data-dependent FT-MS/MS acquisition (R = 15,000 FHMW, 40% HCD) at the Centre for Omic Sciences (COS), Joint Unit of the Universitat Rovira i Virgili and Eurecat18. Labeled and multiplexed peptides (pool labelled with TMT-126 tag) were loaded on a trap nano-column (100 μm I.D.; 2 cm length; 5 μm particle diameter, ThermoFisher Scientific, CA, USA) and separated onto a C18 reversed phase (RP) nano-column (75 μm I.D.; 15 cm length; 3 μm particle diameter, Nikkyo Technos Co. LTD, Japan) on an EASY-II nanoLC from Thermo Fisher. The chromatographic separation was performed using Milli-Q water (0.1% formic acid) and acetonitrile (0.1% formic acid) as mobile phase at 300 nL/min flow rate. Protein identification and quantification were performed using Proteome Discoverer software v.1.4.0.288 (ThermoFisher Scientific, CA, USA) by Multidimensional Protein Identification Technology (MudPIT), combining the two raw data from each sample. For protein identification, all MS and MS/MS spectra were analyzed using the Mascot search engine (v.2.5) combing Homo sapiens (74,449 entries) and contaminants (247 entries) databases. Two missed cleavages and an error of 0.02 Da for FT-MS/MS fragmentation mass and 10.0 ppm for a FT-MS parent ion mass were allowed. Protein quantification was based on the ratios between each TMT-label against-TMT label, with normalization to the protein median.
Metabolomics
Samples were analyzed on a 7200 GC-qTOF from Agilent Technologies (Santa Clara, CA, USA) at the COS18. The chromatographic separation was based on Fiehn Method, using a J&W Scientific HP5-MS (30 m × 0.25 mm i.d., 0.25 μm film capillary column and helium as carrier gas using an oven program from 60 to 325 °C). Ionization was performed by electronic impact (EI) at 70 eV in full scan mode. Metabolites were identified by pure standards or by matching their EI mass spectra and retention time to the metabolomic Fiehn library (from Agilent). Semi-quantification was based on internal standard response ratios.
For the hydrophobic lipids extraction, liquid-liquid extraction based on the Folch procedure was used18. Samples were analyzed on a 1290 Infinity UHPLC coupled to a 6550 qTOF mass spectrometer from Agilent Technologies (Santa Clara, CA, USA) at the COS. Lipid species identification was performed by matching their accurate mass and tandem mass spectrum, when available, to Metlin-PCDL from Agilent. Chromatographic behavior of pure standards for each family and bibliographic data were used to ensure their putative identification. Lipid species were semi-quantified using an internal standard for each lipid family.
Multiomics analyses
A total of 429 molecules were identified, including 239 proteins and 190 metabolites (including lipid species). Several quality control steps were applied, including sample integrity assessment, detection of outliers, and normalization to correct for potential technical effects. Principal component analysis (PCA) was evaluated to ensure group homogeneity. Before statistical analysis, data normalization by median-based correction for systematic differences, Log10 transformation, auto scaling (mean-centered and divided by the standard deviation of each variable) were performed using MetaboAnalyst 5.0. Features with > 50% of missing values were removed and replaced by the median. Omics data were jointly integrated and analyzed in MetaboAnalyst 5.0 for Random Forest, Heatmap, Gene Ontology (GO) enrichment and Joint Pathway analysis based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.
Statistical analysis
Given the sample size (< 30) in the HT group, non-parametric test was applied to be more rigorous for meaningful differences detection between the HT and nHT groups. The distribution of severity groups (mild, severe and critical) between HT and nHT patients was evaluated by the Kruskall-Wallis test. Comparisons between groups were performed using the Chi-square (χ2) test or the Mann–Whitney U test, as requested (IBM SPSS Statistics 26.0). Boxplot graph of selected biomolecules was generated with GraphPad Prism 9.0. Protein interactions networks were generated with STRING 11.5 software. Correlations were analyzed using the Spearman rank coefficient or the point-biserial correlation coefficient, as requested (IBM SPSS Statistics 26.0). Complex figures were created with BioRender.com. The results were considered statistically significant at P ≤ 0.05.
Results
Study characteristics
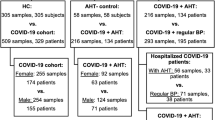
The study included 103 SARS-CoV-2-infected patients classified into mild, severe and critical (Fig. 1A). Among the 103 patients, 26 were HT and 77 were nHT (Fig. 1B). COVID-19 severity was significantly related to the hypertension group, as evaluated by the distribution of the three severity groups (mild, severe and critical) between HT and nHT patients (Fig. 1B, P = 0.008). Given the limited sample size in the HT mild group (n = 4/26) and to focus on the relationship between hypertension and COVID-19 severity, both HT and nHT mild patients were excluded from the analyses (Fig. 1C).
Study characteristics. (A) COVID-19 cohort consisted of 103 patients according to COVID-19 WHO severity23. (B) The cohort was divided according to the presence of hypertension following WHO criteria into HT (n = 26) and nHT (n = 77). (C) The most unfavorable COVID-19 patients (severe and critical) were grouped to evaluate the relation between HT and COVID severity, and both HT and nHT mild groups were excluded from the study. Study characteristics of each group were shown. Created with BioRender.com. HT hypertension, nHT non-hypertension. P values ≤ 0.05 were considered statistically significant.
The hypothesis-generating cohort for the study (Fig. 1C), including the most unfavorable COVID-19 patients (n = 63), comprised 22 (34.9%) HT patients compared to 41 (65.1%) nHT patients. The median age was significantly higher in the HT group (P < 0.001) and most of them were men (P = 0.025). The presence of previous cardiovascular events (P = 0.014), obesity (P = 0.001) or lack of exercise (P = 0.019), predominated in the HT group. Oxygen requirement due to COVID-19 disease was highly prevalent in HT compared to nHT (P = 0.004).
Compounds connecting unfavorable COVID-19 progression to hypertension
First, a heatmap was generated to visualize the molecules significantly different between HT and nHT (Fig. 2A). A total of 27 molecules were increased (red) and 16 metabolites, concretely triglycerides (TG) and phospholipids (PC), were decreased (blue) in HT compared to nHT. These results highlighted the key role of lipids in COVID-19 severity.
Biomolecules related to unfavorable COVID-19 progression in hypertension. (A) Heatmap, created by MetaboAnalyst 5.0, shows the 43 molecules significantly different (non-parametric Mann–Whitney test, P values ≤ 0.05) between the HT and nHT. Mean values for each compound (rows) in each group (columns) are colour-coded based on relative abundance, blue for low) and red for high. (B) RF variable importance plot shows the 15 molecules ranked by classification accuracy to distinguish between HT and nHT. The colours represent the accuracy power of the group (red for high and blue for low). The Y-axis indicates the name of the compounds and the X-axis the mean decreased accuracy (from 0.000 to 0.030). Myo-inositol, PC 32:1 and P06396 (gelsolin) showed the best feature importance in COVID-19 severity in HT. (C) Boxplot of the top three molecules (myo-inositol, PC 32:1 and gelsolin) from RF variable importance plot (B) for each group Y-axis indicates the relative abundance (from 0 to 2) and X-axis the name of the compounds. The horizontal line in each boxplot indicates the mean ± SEM value. (D) ROC curves of the three best molecules obtained in RF (B) to discriminate HT from nHT using IBM SPSS Statistics 21.0. The combination of three molecules was also evaluated (orange line, AUC = 0.746). P values ≤ 0.05 were considered statistically significant.
Next, a machine learning algorithm (Random Forest, RF) was applied to measure which molecules were the most influential in classifying unfavorable COVID-19 progression within the context of hypertension. Myo-inositol (metabolite), phosphatidylcholine 32:1 (PC 32:1) (lipid species) and gelsolin (P06396) (protein) were identified as the most impactful molecules in the RF variable importance plot (Fig. 2B). Myo-inositol and gelsolin were confirmed to be significantly increased in HT (0.122 ± 0.120 and 0.919 ± 0.387, respectively) compared to nHT (0.074 ± 0.034 and 0.681 ± 0.332, respectively). PC 32:1, by contrast, was significantly decreased in HT compared to nHT (0.313 ± 0.218 in HT vs. 0.522 ± 0.396 in nHT) (Fig. 2C). No significant correlation was observed among myo-inositol, PC 32:1 and gelsolin, confirming that changes in one molecule did not predict any change in the other ones. Finally, we examined the relationships between the selected molecules and other important clinical characteristics. Point-biserial correlation analysis discarded any association between the three molecules and the potential confounding factors differentiating HT and nHT (Fig. 1C). Only plasma myo-inositol was significantly correlated with diabetes mellitus (rpb = 0.416; P = 0.035). These findings reinforce the important role of myo-inositol, PC 32:1 and gelsolin in COVID-19 severity, positioning them as potential biomarker for unfavorable COVID-19 progression in HT patients.
Finally, the discriminatory capacity of selected biomarkers to identify high-risk COVID-19 patients was analyzed by binary logistic regression. The combination of the three biomarkers demonstrated the best discriminatory power for HT and nHT due to their high specificity and sensitivity rather than considering them individually (AUC = 0.746, Fig. 2D).
Biological mechanisms related to unfavorable COVID-19 in hypertensives
Bioinformatic analyses were performed to search for and visualize altered pathways in HT vs. nHT in the context of unfavorable COVID-19 progression. First, a protein-protein interaction (PPI) network was created using the STRING tool, which maps known and predicted protein-protein interactions. The network was generated by uploading proteins that exhibited significant differences between groups (Fig. 2A) and adding five additional proteins based on their physical (direct) or functional (indirect) interactions (Fig. 3A). The network view (Fig. 3A) displayed 13 nodes (proteins) and 44 edges (predicted functional associations), with the different colored lines indicating the type of evidence used in predicting the interactions. The resulting network had a PPI enrichment P-value of < 1.0e-16, which indicated that the nodes were not randomly selected and that the observed number of edges was significant. Moreover, functional association analysis revealed that the most involved processes were stress response (biological process), complement and coagulation cascades and protein-lipid complex (local network cluster).
(A) Representation of protein interconnection by STRING. The colors of circles (nodes) indicate clusters of proteins participating in the same biological process or complex. Significant proteins (P values ≤ 0.05) were determined by the non-parametric Mann–Whitney test. Connections of GSN with FGA and A1B were from curated databases and text-mining (B) GO enrichment analysis of the significant biological process (BP) of proteins. The number and percentage of genes encoding proteins (P values) involved in biological processes were indicated for each BP. Y-axis indicates significant BP and X-axis percent of genes (from 0 to 70%). (C) Joint-pathway analysis representing metabolic pathways sorted by pathway impact (pathway topology analysis) (X-axis) and -log10 (p) (pathway enrichment analysis) (Y-axis). Each bubble represents a unique KEGG pathway42; the size of the bubbles shows the pathway impact value (a large bubble represents greater enrichment), and the color denotes the level of significance by means of p-values (darker colors represent the most significant). Only pathways with a P value ≤ 0.05 and an impact ≥ 0.05 were considered significant, and the name was displayed in the representation. The table includes values of a match (number of metabolites in pathway/number of total metabolites in the pathway), impact, P-value and -log10 (p). A1BG alpha-1-B glycoprotein, DG diglyceride, FGA fibrinogen alpha chain, GSN gelsolin, HT hypertension, nHT non-hypertension, PC phosphatidylcholine, SM sphingomyelin, TG triglyceride.
In addition, gene ontology (GO) enrichment analysis was employed to determine whether biological processes, molecular functions or cellular components were associated with COVID-19 severity in hypertensive patients. This analysis revealed that humoral immune response, defence response, regulation of immune response, adaptative immune response and blood coagulation, among others, were biological processes potentially altered in HT patients with unfavorable COVID-19 progression (Fig. 3B). Notably, gelsolin, the protein most highly connected to unfavorable COVID-19 progression in HT (Fig. 2B), was specifically implicated in the stress response (Fig. 3A). Gelsolin (GSN) was connected (via text-mining) with alpha-1-B glycoprotein (A1BG), a protein that was significantly increased in HT (P = 0.02) (Fig. 2A, P04217), and with fibrinogen alpha chain (FGA), an additional protein in the network that was also related to the stress response, complement, coagulation and lipoprotein processes (Fig. 3A).
The multi-omics data was integrated to understand the changes in HT patients with unfavorable COVID-19 progression by joint-pathway analysis. All differentially abundant molecules (Fig. 2A) were pooled into a single query for KEGG pathway representation, with pathway impact on the x-axis and P-value (-log10) on the y-axis. The top contributing pathways (top right corner) included ascorbate and aldarate metabolism (P = 0.021), linoleic acid metabolism (P = 0.028), alpha-linolenic acid metabolism (P = 0.036) and glycosylphosphatidylinositol (GPI)-anchor biosynthesis (P = 0.049) with a P-value < 0.05 (pathway enrichment) and pathway impact ≥ 0.05 (pathway topology) (Fig. 3C). Notably, myo-inositol, the molecule most closely linked to HT in unfavorable COVID-19 progression (Fig. 2B), was the only molecule related to the most significantly changed pathway, ascorbate and aldarate metabolism (impact 0.083, -log(P) = 1.675). These results confirm the direct involvement of myo-inositol in the biological process of stress response, including oxidative stress.
Discussion
Hypertension affects 1.28 billion adults globally, with nearly half unaware of their condition24. As the most common cardiovascular comorbidity in SARS-CoV-2 infection, hypertension significantly increases the risk of severe outcomes, including hospitalization and death2. It is still unclear how hypertension could have a direct impact on the severity of COVID-19, although multiple complex mechanisms, such as RAAS, inflammation and immune responses could be involved10,11,25,26. A previous population-based cohort study identified hypertension as an important independent risk factor for the development of pneumonia and reduced pulmonary function by promoting key factors that may predispose to infection27. Moreover, viral infections trigger an immune response that contributes to elevated blood pressure, thus increasing the susceptibility of hypertensive (HT) patients to serious complications28.
In this study, using an untargeted multi-omics approach, we made a preliminary hypothesis-generating study to improve our understanding of the mechanisms related to COVID-19 severity in HT patients. We sought to identify biomarkers to predict worse clinical outcomes and discover potential therapeutic targets. Most of the biomarkers and metabolic pathways identified were previously associated with either COVID-19 progression18,29,30 or hypertension31,32,33,34. But of interest, we confirm the connection of these molecules and mechanistic pathways with hypertension in unfavorable COVID-19 progression. By including these biomarkers in risk assessment models, clinicians may better identify HT and COVID-19 patients who are at high risk of a worse prognosis and may need closer monitoring.
Several studies have demonstrated an association between hypertension and a worse prognosis for COVID-198,35,36. Consistent with previous reports, severe and critical COVID-19 patients predominated in HT group, while mild cases were more present in non-hypertensive (nHT) group2,25,37,38. Additionally, our HT cohort consisted of men in their 60s who had multiple comorbidities. In agreement, a previous meta-analysis including data from 30 different relevant studies identified elderly males as more likely to develop severe COVID-1939. Age and sex differences in innate immune responses may stem from how innate immunity cells react to inflammatory stimuli, influenced by the X chromosome and sex hormones40,41. Thus, elderly males may be more susceptible to infections and disease severity. On the other hand, a multicenter study has linked hypertension to an increased risk of all-cause mortality, regardless of the presence of other comorbidities8. All these results reinforce that the coexistence of hypertension and SARS-CoV-2 infection causes poorer outcomes and delayed recovery, especially in elderly men.
In the metabolomics approach, myo-inositol was the metabolite that best differentiated HT vs. nHT. Plasma myo-inositol was significantly increased in HT group. Myo-inositol is a widespread and versatile molecule previously related to vascular smooth muscular contraction in hypertension42 and heart failure43. According to this data, increased plasma myo-inositol levels in HT may be linked to a major production of reactive oxygen species (ROS), which will be involved in impaired vascular function44. Myo-inositol is a precursor for phosphoinositides that regulate Protein Kinase C (PKC) activation and influence NADPH oxidase (NOX)-mediated ROS production45. Thus, the oxidative stress that undergoes COVID-19 will be aggravated by hypertension, worsening the clinical situation of hypertensive patients46. Moreover, myo-inositol is a carbocyclic sugar found higher in tissues that use large amounts of glucose and possesses insulin-mimetic properties47,48. Accordingly, it was not surprising that, in our study, plasma myo-inositol levels significantly correlated with diabetes mellitus. In agreement, results from the BElgian and CAnadian MEtabolomics in heart failure with preserved ejection fraction (BECAME-HF) research project identified higher plasma myo-inositol levels in diabetic patients than their non-diabetics, although diabetes and hyperglycaemia alone did not appear to induce myo-inositol elevation43.
Lipidomics is regarded as a subset of metabolomics due to the distinctness and functional specificity of lipids compared to other metabolites. Our lipidomic study demonstrated a distinct lipid profile associated with hypertension. Specifically, plasma triglycerides (TGs) were higher in nHT than HT patients. Accordingly, a decrease in TGs levels was previously related to COVID-19 severity in HT patients, linking it to an elevated inflammatory response49. Moreover, phosphatidylcholine (PC) 32:1, a lipid species that serves as a source of multiple cellular signaling molecules32, was significantly lower in HT patients. PC is the most abundant phospholipid in mammalian cells, playing a key role in regulating lipid, lipoprotein and energy metabolism. Small changes in PC levels can have large implications for metabolic syndrome50. In accordance with our data, Shogli et al.34 proposed PC 32:1 as biomarkers for hypertension because the imbalance of PC levels contributes to hypertension development and are associated with higher mortality in patients with pulmonary arterial hypertension32. Overall, lipids are critical in the SARS-CoV-2 entry to the host, and their dysregulation can cause an alteration of lipid metabolism, aggravating disease outcomes49,51,52. The SARS-CoV-2 entry induces the excessive immune activation (cytokine storm), which causes a dysregulation of lipids production53, thereby worsening the clinical condition of HT patients. Indeed, orlistat, a fatty acid synthase (FASN) inhibitor, and metformin, an AMP-activated protein kinase (AMPK) activator, may effectively suppress coronavirus replication and reduce systemic inflammation, supporting immune homeostasis54. Consequently, the regulation of plasma lipids is crucial for mitigating the risk of poor prognosis.
Beyond lipid metabolism, plasma gelsolin (GSN), an actin-modulating protein, was found significantly higher in HT group. GSN is present in most human tissues and involved in many physiological processes related to pathological and beneficial processes55,56. Elevated circulating gelsolin was found in some types of cancers, heart failure and HIV infection56. Regarding virus infection, of note, increased gelsolin levels were found in HIV-associated dementia (HAD) induced likely by increased viral infection57. Gelsolin possesses five free thiol groups, which are prone to oxidation and reduction reactions. Thus, increased expression of gelsolin in response to oxidative stress may be protective and/or harmful depending on the conditions58. In this line, gelsolin was established as a major determinant in biomechanical stress-mediated advanced heart failure. For example, in heart failure, the gene silencing of GSN could be applied to ameliorate pathological cardiac hypertrophy through the p38/GATA4 signaling pathway59. Furthermore, protein-protein interaction analysis confirms the gelsolin connection with other proteins involved in complement and coagulation pathways. Accordingly, we previously defined proteomic signature related to complement function and coagulation in unfavorable COVID-19 outcomes18. In agreement, a recent study provided evidence of how gelsolin regulates microvesicles (MVs) generation from activated platelets, potentially affecting their pro-inflammatory and procoagulant functions30. This is particularly relevant in COVID-19, as platelet hyperactivation leads to excessive MVs release, contributing to coagulation, thrombosis and microvascular dysfunction60. Due to its involvement in various diseases, gelsolin has been proposed as a potential biomarker for inflammatory conditions56. Gene ontology (GO) enrichment analysis identified the regulation of immune system processes, including both humoral and adaptative immune response, defense response, and blood coagulation as biological processes potentially altered in the HT group. The immune system’s response to SARS-CoV-2 infection is a critical factor in disease progression61,62. The innate immune system includes the component system and is the first line immunological fight against SARS-CoV-2. It may cause the infection to progress and exacerbate inflammation. An excessive complement system activation has been associated with endothelial cell dysfunction, chronic and acute inflammation, and intravascular coagulation. In severe COVID-19 cases, immunothrombosis (innate immune and coagulation systems interaction) dysregulation has been associated with coagulopathy63. Adaptative immune responses, the second line for immunological clearance of SARS-CoV-2, are a critical immune system component for infected host cell destruction and the production of virus-specific antibodies.
Finally, a joint-pathway (pathway enrichment and topology) analysis identified ascorbate and aldarate metabolism and linoleic acid metabolism as the most contributing pathways in HT patients in the context of COVID-19 progression. First, in agreement, a previous study by He et al.33 associated metabolites of ascorbate and aldarate metabolism with blood pressure phenotypes whereas Paul T. et al.29 described ascorbate and aldarate metabolism as one of the pathways enriched in COVID-19 disease. Ascorbate and aldarate metabolism is essential for tissue protection against oxidative stress. This metabolic pathway maintains the reduced state of some enzymes and reacts with oxygen free radicals and lipid peroxidation products to mitigate the damage caused by high oxidative stress64,65. In this sense and closely related to hypertension, ascorbate and aldarate metabolism was associated with renal oxidative stress injury induced by a high-fat diet in obese mice65. Moreover, in psoriasis and psoriatic arthritis patients64, upregulation of ascorbate and aldarate metabolism has been related to immunometabolism reprogramming in circulating Tregs positively linked with TGF and HIF-1 signaling pathways. The severity of COVID-19 infection may be increased by the reduction and exhaustion of T cells in patients61. Second, phosphatidylcholines are phospholipids composed of a choline head group and glycerophosphoric acid, with two fatty acid chains. Thus, the decrease in PC 32:1 could be indirectly related to the alterations in the linoleic acid metabolism. Linoleic acid, a polyunsaturated fatty acid, has a key role in various chronic inflammatory and autoimmune diseases related to preventing microbial infection66. SARS-CoV-2 spike protein receptor binding domain could bind tightly to linoleic acid reducing the interaction and binding of the spike protein to ACE2.
Our study had some limitations. The study cohort was relatively small due to the difficulty of finding unvaccinated COVID-19 patients. Nonetheless, the study was designed to be a preliminary or hypothesis-generating study. This means that it is not possible to statistically calculate the sample size since the number of variables and their variability are unknown in advance. This uncertainty is, in fact, one of the significant advantages of these strategies. Mild cases were excluded from the statistical analyses to focus on the relation between hypertension and COVID-19 severity and to avoid a bias in the study outcomes. Only four hypertensive patients progress to mild COVID-19. No regression model was used to adjust for comorbidities or antihypertensive medications as potential confounders related to hypertension. The sample size was too small for robust regression analyses. The models may not accurately represent the underlying relationship between the variables and the results may be unreliable. Any adjustment for a multiplicity of comparisons should penalize any hypothesis or result for the presence of other unrelated hypotheses or results. Nevertheless, a previous cross-sectional, observational, retrospective multicenter study (SEMI-COVID-19 Network) suggested that hypertension is linked to a higher risk for all-cause mortality independently of other comorbidities, sex, and age8. In the same study, prior treatment with ACEIs/ARBs in hypertensive patients was not associated with a higher all-cause mortality risk compared to other antihypertensives. In any case, a more in-depth evaluation of the potential effects of multiple comorbidities and medications in hypertensive COVID-19 patients will be highly valuable. Also, some clinical characteristics or demographic information such as the SARS-CoV-2 strain variant or some patients habits are missing due to the impossibility of collecting data during the COVID-19 health crisis.
Conclusions
There is a clear relationship between hypertension and COVID-19 severity, demonstrating that hypertension worsens COVID-19 progression. Detection of biomolecules and pathways affected by hypertension may be a strategy to improve diagnosis and prevent severe outcomes in hypertensive COVID-19 patients. Myo-inositol, PC 32:1 and gelsolin were identified as key molecules involved in the unfavorable COVID-19 progression of hypertensive patients. Our findings highlight the implication of myo-inositol and gelsolin in stress response, including oxidative stress, which is a key factor in COVID-19 progression. Moreover, lipidomics confirms the critical role of bioactive lipids in cell immunometabolism reprogramming during adaptive immune response to SARS-CoV-2 infection. Future studies increasing the sample size, particularly those including mild cases should be considered. Also, experimental studies aimed to comprehensively validate our findings and strengthen the robustness of the conclusions will be necessary. Although the direct clinical implementation of these findings as a therapeutic approach presents challenges, our results may serve to enhance risk stratification. The therapeutic potential of bioactive lipids and gelsolin to enhance outcomes and quality of life for hypertensive patients with SARS-CoV-2 should be explored in well-designed clinical studies.
Data availability
Data is available on request from the corresponding author.
References
Huang, Y., Yang, C., Xu, X. F., Xu, W. & Liu, S. W. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol. Sin. 41, 1141–1149 (2020).
Peng, M. et al. Role of hypertension on the severity of COVID-19: A review. J. Cardiovasc. Pharmacol. 78, 648–655 (2021).
Nashiry, A., Sarmin Sumi, S., Islam, S., Quinn, J. M. W. & Moni, M. A. Bioinformatics and system biology approach to identify the influences of COVID-19 on cardiovascular and hypertensive comorbidities. Brief. Bioinform. 22, 1387–1401 (2021).
Nakhaie, S. et al. The effects of antihypertensive medications on severity and outcomes of hypertensive patients with COVID-19. J. Hum. Hypertens. 37, 511–518 (2023).
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020).
Huang, S. et al. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens. Res. 43, 824–831 (2020).
Tadic, M., Cuspidi, C., Grassi, G. & Mancia, G. COVID-19 and arterial hypertension: Hypothesis or evidence?. J. Clin. Hypertens. 22, 1120–1126 (2020).
Rodilla, E. et al. Association of hypertension with all-cause mortality among hospitalized patients with COVID-19. J. Clin. Med. 9, 1–12 (2020).
Okumura, T. & Murohara, T. The renin-angiotensin-aldosterone system inhibitor dilemma in COVID-19: balancing cardiovascular benefits and viral risks. Hypertens. Res. 47, 2598–2600 (2024).
Batiha, G. E. S. et al. Hypertension and its management in COVID-19 patients: The assorted view. Int. J. Cardiol. Cardiovasc. Risk Prev. 11, 200121 (2021).
Onohuean, H. et al. Covid-19 and development of heart failure: mystery and truth Naunyn. Schmiedebergs. Arch. Pharmacol. 394, 2013–2021 (2021).
Ribeiro, A. C. & Uehara, S. C. D. S. A. Systemic arterial hypertension as a risk factor for the severe form of covid-19: scoping review. Rev. Saude Publica 56, 20 (2022).
Samprathi, M. & Jayashree, M. Biomarkers in COVID-19: An up-to-date review. Front. Pediatr. 8, 607647 (2021).
Stefanini, G. G. et al. Early detection of elevated cardiac biomarkers to optimise risk stratification in patients with COVID-19. Heart 106, 1512–1518 (2020).
Qin, J. J. et al. Redefining cardiac biomarkers in predicting mortality of inpatients with COVID-19. Hypertension 76, 1104–1112 (2020).
Shilin, D. S. & Shapovalov, K. G. Changes in some vascular biomarkers in patients with severe COVID-19 with various degrees of pulmonary hypertension. Bull. Exp. Biol. Med. 173, 433–436 (2022).
Ma, J., Deng, Y., Zhang, M. & Yu, J. The role of multi-omics in the diagnosis of COVID-19 and the prediction of new therapeutic targets. Virulence 13, 1101–1110 (2022).
Sánchez, A. et al. Mitochondrial dysfunction, lipids metabolism, and amino acid biosynthesis are key pathways for COVID-19 recovery. iScience 26, 107948 (2023).
Reverté, L. et al. Fetuin-A, inter-α-trypsin inhibitor, glutamic acid and ChoE (18:0) are key biomarkers in a panel distinguishing mild from critical coronavirus disease 2019 outcomes. Clin. Transl. Med. 12, e704 (2022).
Vlasova, S. T., Louis, I., Fang, D., Amer, Y. & Mohei, H. COVID-19-omics report: from individual omics approaches to precision medicine. Reports 6, 45 (2023).
Sood, T. et al. Biomarkers associated with severe COVID-19 among populations with high cardiometabolic risk: A 2-sample mendelian randomization study. JAMA Netw. Open 6, e2325914 (2023).
Wang, H. Diagnosis and treatment protocol for COVID-19 patients (tentative 8th edition). Infect. Dis. Immun. 1, 8–16 (2021).
World Health Organization (WHO). Country & Technical Guidance—Coronavirus disease (COVID-19). https://www.who.int/es/emergencies/diseases/novel-coronavirus-2019/technical-guidance (2020).
World Health Organization (WHO). WHO Hypertension Dashboard. https://www.who.int/news-room/fact-sheets/detail/hypertension (2023).
Muhamad, S. A. et al. COVID-19 and hypertension: The what, the why, and the how. Front. Physiol. 12, 1–11 (2021).
Al-kuraishy, H. M. et al. Covid-19-induced dysautonomia: A menace of sympathetic storm. ASN Neuro 13, 1–10 (2021).
Zekavat, S. M. et al. Elevated blood pressure increases pneumonia risk: epidemiological association and mendelian randomization in the UK biobank. Med 2, 137-148.e4 (2021).
Savedchuk, S., Raslan, R., Nystrom, S. & Sparks, M. A. Emerging viral infections and the potential impact on hypertension, cardiovascular disease, and kidney disease. Circ. Res. 130, 1618–1641 (2022).
Paul, T. et al. Adrenal tropism of SARS-CoV-2 and adrenal findings in a post-mortem case series of patients with severe fatal COVID-19. Nat. Commun. 13, 1589 (2022).
Paul, M., Hong, F., Falet, H. & Kim, H. Gelsolin controls the release of phosphatidylserine (PS)-positive microvesicles (MVs) from platelets. Cell. Signal. 124, 3–6 (2024).
Abou-Saleh, H. et al. Inositol 1,4,5-trisphosphate (IP3) receptor up-regulation in hypertension is associated with sensitization of Ca2+ release and vascular smooth muscle contractility. J. Biol. Chem. 288, 32941–32951 (2013).
Wawrzyniak, R. et al. Plasma untargeted metabolomics with proteinase K discloses phospholipid signature associated with pulmonary arterial hypertension. Sci. Rep. 13, 15280 (2023).
He, W. J. et al. An untargeted metabolomics study of blood pressure: Findings from the Bogalusa heart study. J. Hypertens. 38, 1302–1311 (2020).
Shoghli, M. et al. The novel ceramide- and phosphatidylcholine-based risk score for the prediction of new-onset of hypertension. J. Clin. Med. 12, 7524 (2023).
Thakur, B. et al. A systematic review and meta-analysis of geographic differences in comorbidities and associated severity and mortality among individuals with COVID-19. Sci. Rep. 11, 8562 (2021).
Gallo, G., Calvez, V. & Savoia, C. Hypertension and COVID-19: Current evidence and perspectives. High Blood Press. Cardiovasc. Prev. 29, 115–123 (2022).
Trump, S. et al. Hypertension delays viral clearance and exacerbates airway hyperinflammation in patients with COVID-19. Nat. Biotechnol. 39, 705–716 (2021).
Bermejo-Martin, J. F. et al. Effect of viral storm in patients admitted to intensive care units with severe COVID-19 in Spain: a multicentre, prospective, cohort study. Lancet Microbe 4, e431–e441 (2023).
Hu, J. & Wang, Y. The clinical characteristics and risk factors of severe COVID-19. Gerontology 67, 255–266 (2021).
Chuang, L. & Tambyah, P. A. Catheter-associated urinary tract infection. J. Infect. Chemother. 27, 1400–1406 (2021).
Jaillon, S., Berthenet, K. & Garlanda, C. Sexual dimorphism in innate immunity. Clin. Rev. Allergy Immunol. 56, 308–321 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Pouleur, A. C. et al. Plasma myo-inositol elevation in heart failure: clinical implications and prognostic significance. Results from the BElgian and CAnadian MEtabolomics in HFpEF (BECAME-HF) research project. eBioMedicine 107, 105264 (2024).
Touyz, R. M. Reactive oxygen species, vascular oxidative stress, and redox signaling in hypertension: What is the clinical significance?. Hypertension 44, 248–252 (2004).
De Champlain, J. et al. Oxidative stress in hypertension. Clin. Exp. Hypertens. 26, 593–601 (2004).
Vollbracht, C. & Kraft, K. Oxidative stress and hyper-inflammation as major drivers of severe COVID-19 and long COVID: Implications for the benefit of high-dose intravenous vitamin C. Front. Pharmacol. 13, 899198 (2022).
Croze, M. L. & Soulage, C. O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 95, 1811–1827 (2013).
DiNicolantonio, J. J. & H O’Keefe, J. Myo-inositol for insulin resistance, metabolic syndrome, polycystic ovary syndrome and gestational diabetes. Open Hear. 9, e001989 (2022).
Elrayess, M. A. et al. Metabolic signatures of type 2 diabetes mellitus and hypertension in COVID-19 patients with different disease severity. Front. Med. 8, 788687 (2022).
van der Veen, J. N. et al. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 1859, 1558–1572 (2017).
Theken, K. N., Tang, S. Y., Sengupta, S. & FitzGerald, G. A. The roles of lipids in SARS-CoV-2 viral replication and the host immune response. J. Lipid Res. 62, 100129 (2021).
Wrona, M. & Skrypnik, D. New-onset diabetes mellitus, hypertension, dyslipidaemia as sequelae of COVID-19 infection—Systematic review. Int. J. Environ. Res. Public Health 19, 13280 (2022).
Sorokin, A. V. et al. COVID-19—Associated dyslipidemia: Implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. 34, 9843–9853 (2020).
Tanner, J. E. & Alfieri, C. The fatty acid lipid metabolism nexus in COVID-19. Viruses 13, 90 (2021).
Feldt, J. et al. Structure, regulation and related diseases of the actin-binding protein gelsolin. Expert Rev. Mol. Med. 20, 1–10 (2019).
Piktel, E., Levental, I., Durnaś, B., Janmey, P. A. & Bucki, R. Plasma gelsolin: Indicator of inflammation and its potential as a diagnostic tool and therapeutic target. Int. J. Mol. Sci. 19, 2516 (2018).
Wiederin, J., Rozek, W., Duan, F. & Ciborowski, P. Biomarkers of HIV-1 associated dementia: Proteomic investigation of sera. Proteome Sci. 7, 1–12 (2009).
Li, G. H., Arora, P. D., Chen, Y., Mcculloch, C. A. & Liu, P. Multifunctional roles of gelsolin in health and diseases. Med. Res. Rev. 32, 999–1025 (2012).
Hu, W. S. et al. Gelsolin (GSN) induces cardiomyocyte hypertrophy and BNP expression via p38 signaling and GATA-4 transcriptional factor activation. Mol. Cell. Biochem. 390, 263–270 (2014).
Zaid, Y. & Guessous, F. The ongoing enigma of SARS-CoV-2 and platelet interaction. Res. Pract. Thromb. Haemost. 6, e12642 (2022).
Mohammed, R. N. et al. A comprehensive review about immune responses and exhaustion during coronavirus disease (COVID-19). Cell Commun. Signal. 20, 79 (2022).
Guo, Z. Y. et al. COVID-19: from immune response to clinical intervention. Precis. Clin. Med. 7, pbae015 (2024).
Loo, J., Spittle, D. A. & Newnham, M. COVID-19, immunothrombosis and venous thromboembolism: Biological mechanisms. Thorax 76, 412–420 (2021).
Peng, L. et al. Single-cell transcriptomic landscape of immunometabolism reveals intervention candidates of ascorbate and aldarate metabolism, fatty-acid degradation and PUFA metabolism of T-cell subsets in healthy controls, psoriasis and psoriatic arthritis. Front. Immunol. 14, 1–16 (2023).
Liang, H. & Song, K. Elucidating ascorbate and aldarate metabolism pathway characteristics via integration of untargeted metabolomics and transcriptomics of the kidney of high-fat diet-fed obese mice. PLoS One 19, 1–19 (2024).
Das, U. N. Essential fatty acids and their metabolites in the pathobiology of inflammation and its resolution. Biomolecules 11, 1873 (2021).
Acknowledgements
The authors acknowledge the collaboration of all the patients and their families and medical and nursing staff who have taken part in the project; the Departments of Preventive Medicine and Epidemiology, Internal Medicine, Critical Care, Emergency, Occupational Health, Laboratory Medicine and Molecular Biology; and BioBank-IISPV (B.0000853 + B.0000854) integrated into the Spanish National Biobanks Platform (PT20/00197), CERCA Program (Generalitat de Catalunya) and IISPV. Authors thank specialized technicians of proteomic and metabolomics facility of the Centre for Omic Sciences (COS) Joint Unit of the Universitat Rovira i Virgili-Eurecat for their contribution to mass spectrometry analysis.
Funding
This work has been developed in the framework of the COVIDOMICS’ project supported by Direcció General de Recerca i Innovació en Salut (DGRIS), Departament de Salut, Generalitat de Catalunya (PoC-6-17). The research was also supported by the Programa de Suport als Grups de Recerca AGAUR (2021SGR01404) and Universitat Rovira i Virgili (AJUT URV 2022PFR-URV-59), and the CIBER -Consorcio Centro de Investigación Biomédica en Red- (CB07/08/0012, CB21/13/00020, CB21/13/00063), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación and Unión Europea—NextGenerationEU. A.R. is supported by Instituto de Salud Carlos III (ISCIII) under grant agreement ‘‘CP19/00146’’ through the Miguel Servet Program, A.S. by Unión Europea—Next Generattion EU (grant “2022 INV-1 00036” of Investigo Program), and L.C. by the grant PID2020-119030RJ-I00 financed by MCIN/AEI/https://doi.org/10.13039/501100011033.
Author information
Authors and Affiliations
Contributions
Conceptualization—A.R., J.P., FV; Data Curation—A.R., J.P.; Acquisition, analysis or interpretation of data—A.S., G.G.; F.G., A.M., S.C., M.F.; Funding Acquisition—A.R., F.V.; Supervision—A.R., J.P. F.V.; Drafting of the manuscript—A.S., G.G., A.R.; Critical revision of the manuscript—F.V., L.C., M.M. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics declarations
Protocols were carried out following the recommendations of the Ethical and Scientific Committees from each participating institution and were approved by the Committee for Ethical Clinical Research following the rules of Good Clinical Practice from the IISPV (079/ 2020, CEIm IISPV). The CEIm IISPV is an independent committee made up of health and non-health professionals who supervise the correct compliance of the ethical principles governing clinical trials and research projects that are performed in our environment, specifically in their methodology, ethics, and laws. All subjects or their relatives gave written informed consent following the Declaration of Helsinki.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sánchez, A., García-Pardo, G., Martí, A. et al. Omics for searching plasma biomarkers associated with unfavorable COVID-19 progression in hypertensive patients. Sci Rep 15, 10343 (2025). https://doi.org/10.1038/s41598-025-94725-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-025-94725-4